Vasodilator-stimulated phosphoprotein: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
==Structural highlights == | ==Structural highlights == | ||
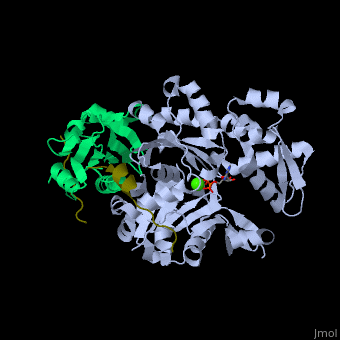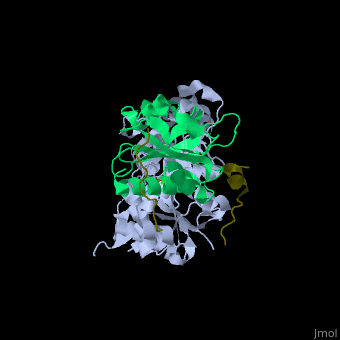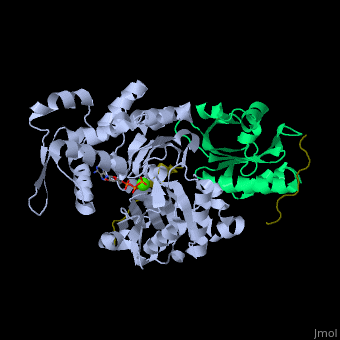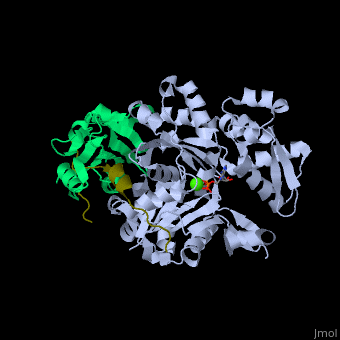
<scene name='50/508423/Cv/9'>Human VASP complex with α-actin, profilin-1, ATP (stick model) and Ca+2 ion</scene> ([[2pbd]]). VASP binds actin with a <scene name='50/508423/Cv/10'>poly-Pro site</scene><ref>PMID:17914456</ref>. | <scene name='50/508423/Cv/9'>Human VASP complex with α-actin, profilin-1, ATP (stick model) and Ca+2 ion</scene> ([[2pbd]]). VASP binds actin with a <scene name='50/508423/Cv/10'>poly-Pro site</scene><ref>PMID:17914456</ref>. | ||
*<scene name='50/508423/Cv/13'>Ca coordination site</scene>. | |||
</StructureSection> | </StructureSection> | ||
==3D structure of vasodilator-stimulated phosphoprotein== | ==3D structure of vasodilator-stimulated phosphoprotein== | ||
Revision as of 17:36, 13 January 2021
FunctionVasodilator-stimulated phosphoprotein (VASP) belongs to the Ena-VASP family. It binds proteins with E/DFPPPPXD/E motif and targets them to focal adhesion cell membranes. VASP is an actin- and profilin-binding protein[1]. VASP is associated with filament formation and promotes elongation. VASP protects the actin barb edge against capping and increases the rate of actin polymerization in the presence of capping protein. VASP is a homotetramer. For more details see Group:MUZIC:Mena_VASP. RelevanceVASP restricts the spread of Shigella which causes diarrhoreal disease[2]. Structural highlights(2pbd). VASP binds actin with a [3].
|
| ||||||||||
3D structure of vasodilator-stimulated phosphoprotein3D structure of vasodilator-stimulated phosphoprotein
Updated on 13-January-2021
1egx – hVASP EVH1 domain – human – NMR
1usd, 1use - hVASP tetramerization domain
2pav, 2pbd, 3chw – hVASP + actin + profilin-1
2v8c – hVASP + profilin-2
ReferencesReferences
- ↑ Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006 Jan 15;393(Pt 2):555-64. PMID:16197368 doi:http://dx.doi.org/BJ20050796
- ↑ Lee SY, Gertler FB, Goldberg MB. Vasodilator-stimulated phosphoprotein restricts cell-to-cell spread of Shigella flexneri at the cell periphery. Microbiology. 2015 Nov;161(11):2149-60. doi: 10.1099/mic.0.000173. Epub 2015 Sep , 9. PMID:26358985 doi:http://dx.doi.org/10.1099/mic.0.000173
- ↑ Ferron F, Rebowski G, Lee SH, Dominguez R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007 Oct 31;26(21):4597-606. Epub 2007 Oct 4. PMID:17914456