1mq8: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
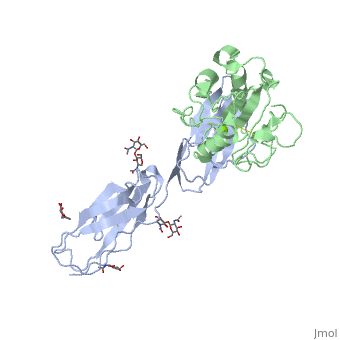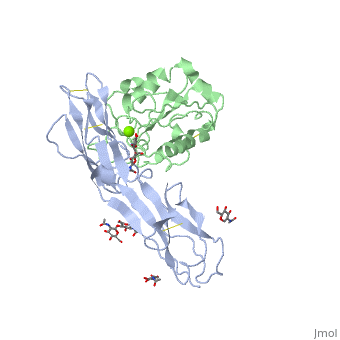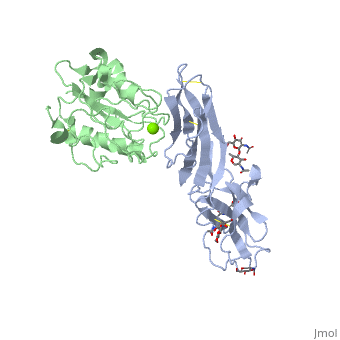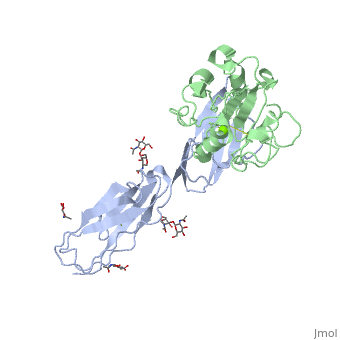
==Crystal structure of alphaL I domain in complex with ICAM-1== | ==Crystal structure of alphaL I domain in complex with ICAM-1== | ||
<StructureSection load='1mq8' size='340' side='right' caption='[[1mq8]], [[Resolution|resolution]] 3.30Å' scene=''> | <StructureSection load='1mq8' size='340' side='right'caption='[[1mq8]], [[Resolution|resolution]] 3.30Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[1mq8]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1MQ8 OCA]. For a <b>guided tour on the structure components</b> use [http:// | <table><tr><td colspan='2'>[[1mq8]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1MQ8 OCA]. For a <b>guided tour on the structure components</b> use [http://proteopedia.org/fgij/fg.htm?mol=1MQ8 FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene></td></tr> | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1mq9|1mq9]], [[1mqa|1mqa]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat"><div style='overflow: auto; max-height: 3em;'>[[1mq9|1mq9]], [[1mqa|1mqa]]</div></td></tr> | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">ICAM-1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN]), LFA-1 (AlphaLbeta2) ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">ICAM-1 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN]), LFA-1 (AlphaLbeta2) ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http:// | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://proteopedia.org/fgij/fg.htm?mol=1mq8 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1mq8 OCA], [http://pdbe.org/1mq8 PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1mq8 RCSB], [http://www.ebi.ac.uk/pdbsum/1mq8 PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=1mq8 ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 15: | Line 15: | ||
Check<jmol> | Check<jmol> | ||
<jmolCheckbox> | <jmolCheckbox> | ||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/mq/1mq8_consurf.spt"</scriptWhenChecked> | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/mq/1mq8_consurf.spt"</scriptWhenChecked> | ||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
| Line 30: | Line 30: | ||
</div> | </div> | ||
<div class="pdbe-citations 1mq8" style="background-color:#fffaf0;"></div> | <div class="pdbe-citations 1mq8" style="background-color:#fffaf0;"></div> | ||
==See Also== | |||
*[[Integrin 3D structures|Integrin 3D structures]] | |||
*[[Intercellular adhesion molecule|Intercellular adhesion molecule]] | |||
== References == | == References == | ||
<references/> | <references/> | ||
| Line 35: | Line 39: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Human]] | [[Category: Human]] | ||
[[Category: Large Structures]] | |||
[[Category: Dong, Y]] | [[Category: Dong, Y]] | ||
[[Category: Joachimiak, A]] | [[Category: Joachimiak, A]] | ||
Revision as of 11:40, 2 December 2020
Crystal structure of alphaL I domain in complex with ICAM-1Crystal structure of alphaL I domain in complex with ICAM-1
Structural highlights
Function[ICAM1_HUMAN] ICAM proteins are ligands for the leukocyte adhesion protein LFA-1 (integrin alpha-L/beta-2). During leukocyte trans-endothelial migration, ICAM1 engagement promotes the assembly of endothelial apical cups through ARHGEF26/SGEF and RHOG activation. In case of rhinovirus infection acts as a cellular receptor for the virus.[1] [2] [3] [4] [ITAL_HUMAN] Integrin alpha-L/beta-2 is a receptor for ICAM1, ICAM2, ICAM3 and ICAM4. It is involved in a variety of immune phenomena including leukocyte-endothelial cell interaction, cytotoxic T-cell mediated killing, and antibody dependent killing by granulocytes and monocytes. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe structure of the I domain of integrin alpha L beta 2 bound to the Ig superfamily ligand ICAM-1 reveals the open ligand binding conformation and the first example of an integrin-IgSF interface. The I domain Mg2+ directly coordinates Glu-34 of ICAM-1, and a dramatic swing of I domain residue Glu-241 enables a critical salt bridge. Liganded and unliganded structures for both high- and intermediate-affinity mutant I domains reveal that ligand binding can induce conformational change in the alpha L I domain and that allosteric signals can convert the closed conformation to intermediate or open conformations without ligand binding. Pulling down on the C-terminal alpha 7 helix with introduced disulfide bonds ratchets the beta 6-alpha 7 loop into three different positions in the closed, intermediate, and open conformations, with a progressive increase in affinity. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation.,Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang JH, Springer TA Cell. 2003 Jan 10;112(1):99-111. PMID:12526797[5] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||