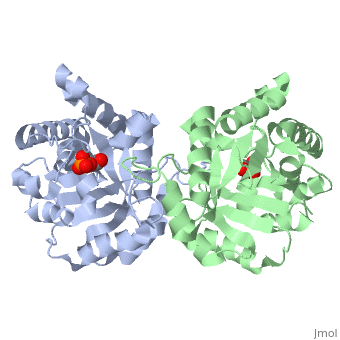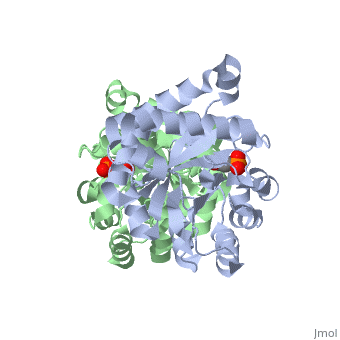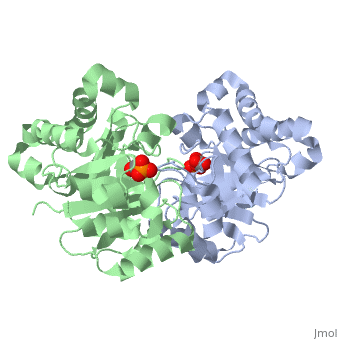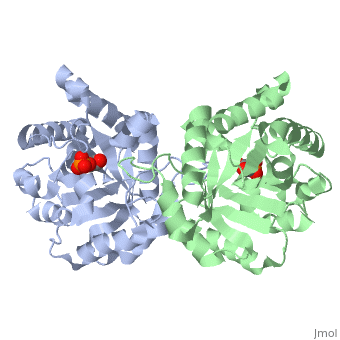2ypi: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==CRYSTALLOGRAPHIC ANALYSIS OF THE COMPLEX BETWEEN TRIOSEPHOSPHATE ISOMERASE AND 2-PHOSPHOGLYCOLATE AT 2.5-ANGSTROMS RESOLUTION. IMPLICATIONS FOR CATALYSIS== | ==CRYSTALLOGRAPHIC ANALYSIS OF THE COMPLEX BETWEEN TRIOSEPHOSPHATE ISOMERASE AND 2-PHOSPHOGLYCOLATE AT 2.5-ANGSTROMS RESOLUTION. IMPLICATIONS FOR CATALYSIS== | ||
<StructureSection load='2ypi' size='340' side='right' caption='[[2ypi]], [[Resolution|resolution]] 2.50Å' scene=''> | <StructureSection load='2ypi' size='340' side='right'caption='[[2ypi]], [[Resolution|resolution]] 2.50Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2ypi]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Atcc_18824 Atcc 18824]. The February 2004 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''The Glycolytic Enzymes'' by David S. Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2004_2 10.2210/rcsb_pdb/mom_2004_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2YPI OCA]. For a <b>guided tour on the structure components</b> use [http:// | <table><tr><td colspan='2'>[[2ypi]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Atcc_18824 Atcc 18824]. The February 2004 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''The Glycolytic Enzymes'' by David S. Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2004_2 10.2210/rcsb_pdb/mom_2004_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2YPI OCA]. For a <b>guided tour on the structure components</b> use [http://proteopedia.org/fgij/fg.htm?mol=2YPI FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=PGA:2-PHOSPHOGLYCOLIC+ACID'>PGA</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=PGA:2-PHOSPHOGLYCOLIC+ACID'>PGA</scene></td></tr> | ||
<tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Triose-phosphate_isomerase Triose-phosphate isomerase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=5.3.1.1 5.3.1.1] </span></td></tr> | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Triose-phosphate_isomerase Triose-phosphate isomerase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=5.3.1.1 5.3.1.1] </span></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http:// | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://proteopedia.org/fgij/fg.htm?mol=2ypi FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ypi OCA], [http://pdbe.org/2ypi PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=2ypi RCSB], [http://www.ebi.ac.uk/pdbsum/2ypi PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=2ypi ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
| Line 30: | Line 30: | ||
==See Also== | ==See Also== | ||
*[[Triose Phosphate Isomerase|Triose Phosphate Isomerase]] | *[[Triose Phosphate Isomerase|Triose Phosphate Isomerase]] | ||
*[[Triose phosphate isomerase 3D structures|Triose phosphate isomerase 3D structures]] | |||
== References == | == References == | ||
<references/> | <references/> | ||
| Line 35: | Line 36: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Atcc 18824]] | [[Category: Atcc 18824]] | ||
[[Category: Large Structures]] | |||
[[Category: RCSB PDB Molecule of the Month]] | [[Category: RCSB PDB Molecule of the Month]] | ||
[[Category: The Glycolytic Enzymes]] | [[Category: The Glycolytic Enzymes]] | ||
Revision as of 16:01, 22 July 2020
CRYSTALLOGRAPHIC ANALYSIS OF THE COMPLEX BETWEEN TRIOSEPHOSPHATE ISOMERASE AND 2-PHOSPHOGLYCOLATE AT 2.5-ANGSTROMS RESOLUTION. IMPLICATIONS FOR CATALYSISCRYSTALLOGRAPHIC ANALYSIS OF THE COMPLEX BETWEEN TRIOSEPHOSPHATE ISOMERASE AND 2-PHOSPHOGLYCOLATE AT 2.5-ANGSTROMS RESOLUTION. IMPLICATIONS FOR CATALYSIS
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe binding of the transition-state analogue 2-phosphoglycolate to triosephosphate isomerase from yeast has been investigated crystallographically. An atomic model of the enzyme-inhibitor complex has been refined against data to 2.5-A resolution to a final R factor of 0.18. The interactions between the inhibitor and enzyme have been analyzed. The inhibitor forms hydrogen bonds to the side chains of His 95 and Glu 165. The latter hydrogen bond confirms that Glu 165 is protonated upon PGA binding. The structure of the complexed enzyme has been compared to that of the unbound form of the enzyme, and conformational changes have been observed: the side chain of Glu 165 moves over 2 A and a 10-residue flexible loop moves over 7 A to close over the active site. Spectroscopic results of phosphoglycolic acid binding to triosephosphate isomerase that have been amassed over the years are also explained in structural terms. The implications for catalysis are noted. Crystallographic analysis of the complex between triosephosphate isomerase and 2-phosphoglycolate at 2.5-A resolution: implications for catalysis.,Lolis E, Petsko GA Biochemistry. 1990 Jul 17;29(28):6619-25. PMID:2204418[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||