Growth factor receptor-bound protein: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
Michal Harel (talk | contribs) No edit summary |
||
| Line 8: | Line 8: | ||
== Structural highlights == | == Structural highlights == | ||
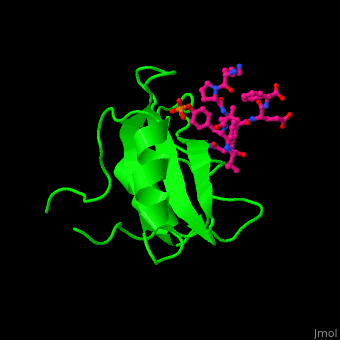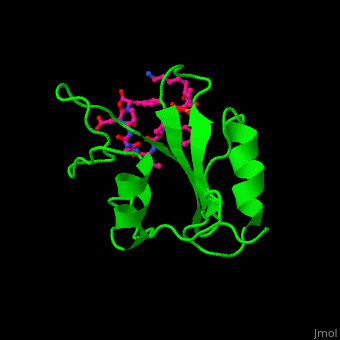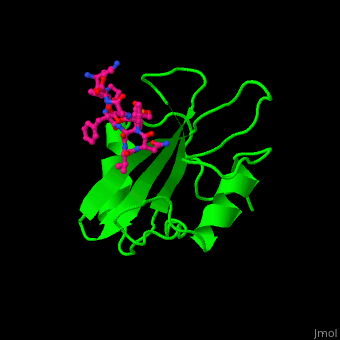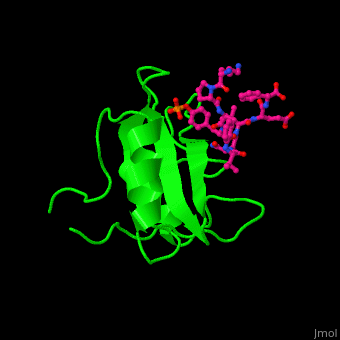
Human GRB2 residue <scene name='51/516448/Cv/5'>Trp121 forces the phosphotyrosyl containing ligand</scene> into a turn conformation. Water molecules are shown as red spheres. <scene name='51/516448/Cv/6'>Phosphotyrosine binding</scene> <ref>PMID:10090780</ref>. | Human GRB2 residue <scene name='51/516448/Cv/5'>Trp121 forces the phosphotyrosyl containing ligand</scene> into a turn conformation. Water molecules are shown as red spheres. <scene name='51/516448/Cv/6'>Phosphotyrosine binding</scene> <ref>PMID:10090780</ref>. | ||
==3D structures of growth factor receptor-bound proteins== | |||
[[Growth factor receptor-bound proteins 3D structures]] | |||
</StructureSection> | </StructureSection> | ||
| Line 19: | Line 23: | ||
**[[1gri]], [[2h46]] – hGRB2 – human <br /> | **[[1gri]], [[2h46]] – hGRB2 – human <br /> | ||
*''GRB2 C terminal SH3 domain'' | *''GRB2 C terminal SH3 domain'' residues 159-214 | ||
**[[1gfc]], [[1gfd]] – hGRB2 C terminal SH3 domain - NMR<br /> | **[[1gfc]], [[1gfd]] – hGRB2 C terminal SH3 domain - NMR<br /> | ||
| Line 26: | Line 30: | ||
**[[1gcq]] – hGRB2 C terminal SH3 domain + VAV proto-oncogene SH3 domain<br /> | **[[1gcq]] – hGRB2 C terminal SH3 domain + VAV proto-oncogene SH3 domain<br /> | ||
*''GRB2 N terminal SH3 domain'' | *''GRB2 N terminal SH3 domain'' residues 1-61 | ||
**[[1gbr]], [[1gbq]], [[2gbq]], [[3gbq]], [[4gbq]] – mGRB2 N terminal SH3 domain + Sos-A peptide – mouse - NMR<br /> | **[[1gbr]], [[1gbq]], [[2gbq]], [[3gbq]], [[4gbq]] – mGRB2 N terminal SH3 domain + Sos-A peptide – mouse - NMR<br /> | ||
**[[1aze]] – mGRB2 N terminal SH3 domain + Sos-A peptide - NMR<br /> | **[[1aze]] – mGRB2 N terminal SH3 domain + Sos-A peptide - NMR<br /> | ||
*''GRB2 SH2 domain'' | *''GRB2 SH2 domain'' residues 60-158 | ||
**[[1jyu]] – hGRB2 SH2 domain <br /> | **[[1jyu]], [[6icg]], [[6ich]] – hGRB2 SH2 domain <br /> | ||
**[[1ghu]] – hGRB2 SH2 domain (mutant) - NMR<br /> | **[[1ghu]] – hGRB2 SH2 domain (mutant) - NMR<br /> | ||
**[[1fhs]] – hGRB2 SH2 domain - NMR<br /> | **[[1fhs]] – hGRB2 SH2 domain - NMR<br /> | ||
| Line 55: | Line 59: | ||
**[[1mw4]], [[2l4k]] – hGRB7 SH2 domain + phosphor-Tyr peptide - NMR<br /> | **[[1mw4]], [[2l4k]] – hGRB7 SH2 domain + phosphor-Tyr peptide - NMR<br /> | ||
**[[3pqz]], [[5u1q]], [[5u06]], [[5tyi]] – hGRB7 SH2 domain + cyclic peptide <br /> | **[[3pqz]], [[5u1q]], [[5u06]], [[5tyi]] – hGRB7 SH2 domain + cyclic peptide <br /> | ||
**[[1wgr]] – hGRB7 RA domain - NMR<br /> | **[[1wgr]] – hGRB7 RA domain residues 100-186 - NMR<br /> | ||
*GRB10 | *GRB10 | ||
| Line 61: | Line 65: | ||
**[[1nrv]] – hGRB10 SH2 domain <br /> | **[[1nrv]] – hGRB10 SH2 domain <br /> | ||
**[[3m7f]] – hGRB10 SH2 domain + NEDD4 C2 domain<br /> | **[[3m7f]] – hGRB10 SH2 domain + NEDD4 C2 domain<br /> | ||
**[[3hk0]] – hGRB10 | **[[3hk0]] – hGRB10 PH domain residues 164-415 <br /> | ||
*GRB14 | *GRB14 | ||
Revision as of 10:52, 18 July 2019
FunctionGrowth factor receptor-bound proteins (GRB) are adaptor proteins. GRBs contain SH2, Ras-associated (RA) and pleckstrin homology (PH) domains.
Structural highlightsHuman GRB2 residue into a turn conformation. Water molecules are shown as red spheres. [4]. 3D structures of growth factor receptor-bound proteinsGrowth factor receptor-bound proteins 3D structures
|
| ||||||||||
3D structures of growth factor receptor-bound proteins3D structures of growth factor receptor-bound proteins
Updated on 18-July-2019
ReferencesReferences
- ↑ Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431-42. PMID:1322798
- ↑ Nadler Y, Gonzalez AM, Camp RL, Rimm DL, Kluger HM, Kluger Y. Growth factor receptor-bound protein-7 (Grb7) as a prognostic marker and therapeutic target in breast cancer. Ann Oncol. 2010 Mar;21(3):466-73. doi: 10.1093/annonc/mdp346. Epub 2009 Aug 28. PMID:19717535 doi:http://dx.doi.org/10.1093/annonc/mdp346
- ↑ Deng Y, Bhattacharya S, Swamy OR, Tandon R, Wang Y, Janda R, Riedel H. Growth factor receptor-binding protein 10 (Grb10) as a partner of phosphatidylinositol 3-kinase in metabolic insulin action. J Biol Chem. 2003 Oct 10;278(41):39311-22. Epub 2003 Jun 3. PMID:12783867 doi:http://dx.doi.org/10.1074/jbc.M304599200
- ↑ Ettmayer P, France D, Gounarides J, Jarosinski M, Martin MS, Rondeau JM, Sabio M, Topiol S, Weidmann B, Zurini M, Bair KW. Structural and conformational requirements for high-affinity binding to the SH2 domain of Grb2(1). J Med Chem. 1999 Mar 25;42(6):971-80. PMID:10090780 doi:10.1021/jm9811007