3dyt: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Crystal structure of Snx9PX-BAR (230-595), C2221== | ==Crystal structure of Snx9PX-BAR (230-595), C2221== | ||
<StructureSection load='3dyt' size='340' side='right' caption='[[3dyt]], [[Resolution|resolution]] 2.08Å' scene=''> | <StructureSection load='3dyt' size='340' side='right' caption='[[3dyt]], [[Resolution|resolution]] 2.08Å' scene=''> | ||
| Line 7: | Line 8: | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[3dyu|3dyu]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[3dyu|3dyu]]</td></tr> | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SNX9, SH3PX1, SH3PXD3A ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">SNX9, SH3PX1, SH3PXD3A ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3dyt FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3dyt OCA], [http://pdbe.org/3dyt PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=3dyt RCSB], [http://www.ebi.ac.uk/pdbsum/3dyt PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3dyt FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3dyt OCA], [http://pdbe.org/3dyt PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=3dyt RCSB], [http://www.ebi.ac.uk/pdbsum/3dyt PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=3dyt ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 15: | Line 16: | ||
Check<jmol> | Check<jmol> | ||
<jmolCheckbox> | <jmolCheckbox> | ||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/dy/3dyt_consurf.spt"</scriptWhenChecked> | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/dy/3dyt_consurf.spt"</scriptWhenChecked> | ||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
Revision as of 12:32, 14 November 2018
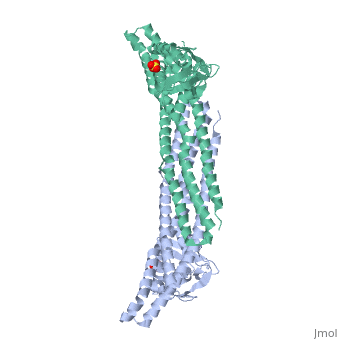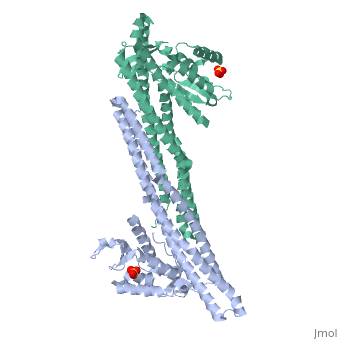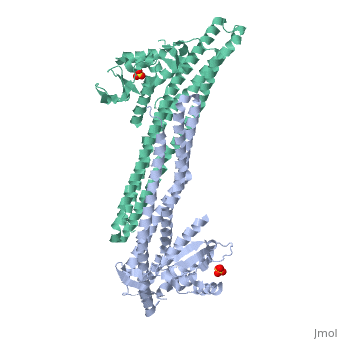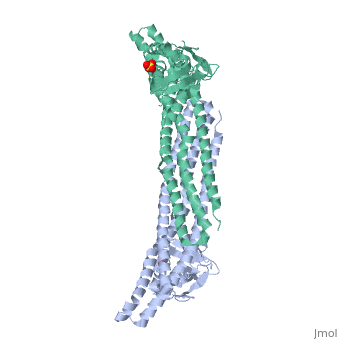
Crystal structure of Snx9PX-BAR (230-595), C2221Crystal structure of Snx9PX-BAR (230-595), C2221
Structural highlights
Function[SNX9_HUMAN] May be involved in several stages of intracellular trafficking. Plays a role in endocytosis via clathrin-coated pits, but also clathrin-independent, actin-dependent fluid-phase endocytosis. Plays a role in macropinocytosis. Promotes internalization of TNFR. Promotes degradation of EGFR after EGF signaling. Stimulates the GTPase activity of DNM1. Promotes DNM1 oligomerization. Promotes activation of the Arp2/3 complex by WASL, and thereby plays a role in the reorganization of the F-actin cytoskeleton. Binds to membranes enriched in phosphatidylinositol 4,5-bisphosphate and promotes membrane tubulation. Has lower affinity for membranes enriched in phosphatidylinositol 3-phosphate.[1] [2] [3] [4] [5] [6] [7] [8] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedEndophilin and Sorting Nexin 9 (Snx9) play key roles in endocytosis by membrane curvature sensing and remodeling via their Bin/Amphiphysin/Rvs (BAR) domains. BAR and the related F-BAR domains form dimeric, crescent-shaped units that occur N- or C-terminally to other lipid-binding, adaptor, or catalytic modules. In crystal structures, the PX-BAR unit of Snx9 (Snx9(PX-BAR)) adopts an overall compact, moderately curved conformation. SAXS-based solution studies revealed an alternative, more curved state of Snx9(PX-BAR) in which the PX domains are flexibly connected to the BAR domains, providing a model for how Snx9 exhibits lipid-dependent curvature preferences. In contrast, Endophilin appears to be rigid in solution, and the SH3 domains are located at the distal tips of a BAR domain dimer with fixed curvature. We also observed tip-to-tip interactions between the BAR domains in a trigonal crystal form of Snx9(PX-BAR) reminiscent of functionally important interactions described for F-BAR domains. Structure and plasticity of Endophilin and Sorting Nexin 9.,Wang Q, Kaan HY, Hooda RN, Goh SL, Sondermann H Structure. 2008 Oct 8;16(10):1574-87. PMID:18940612[9] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||||