2c6g: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
== | |||
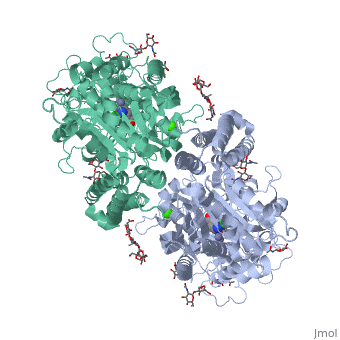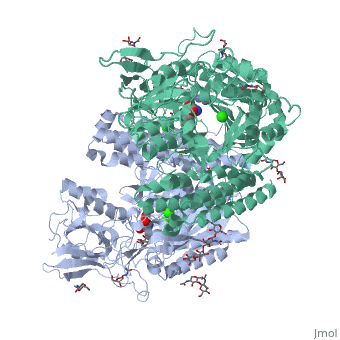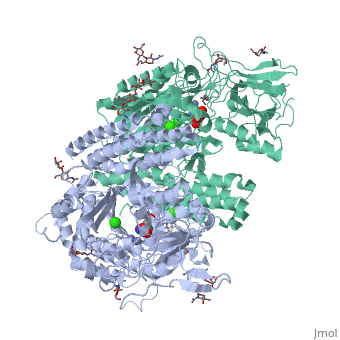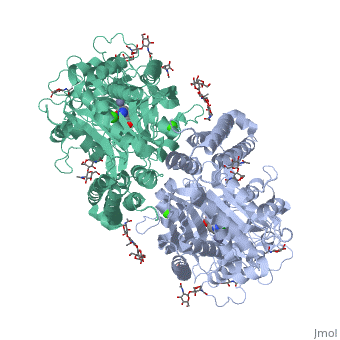
==membrane-bound glutamate carboxypeptidase II (GCPII) with bound glutamate== | |||
<StructureSection load='2c6g' size='340' side='right' caption='[[2c6g]], [[Resolution|resolution]] 2.20Å' scene=''> | <StructureSection load='2c6g' size='340' side='right' caption='[[2c6g]], [[Resolution|resolution]] 2.20Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2c6g]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2C6G OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2C6G FirstGlance]. <br> | <table><tr><td colspan='2'>[[2c6g]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2C6G OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2C6G FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=BMA:BETA-D-MANNOSE'>BMA</scene>, <scene name='pdbligand=CA:CALCIUM+ION'>CA</scene>, <scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene>, <scene name='pdbligand=GLU:GLUTAMIC+ACID'>GLU</scene>, <scene name='pdbligand= | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=BMA:BETA-D-MANNOSE'>BMA</scene>, <scene name='pdbligand=CA:CALCIUM+ION'>CA</scene>, <scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene>, <scene name='pdbligand=GLU:GLUTAMIC+ACID'>GLU</scene>, <scene name='pdbligand=MAN:ALPHA-D-MANNOSE'>MAN</scene>, <scene name='pdbligand=NAG:N-ACETYL-D-GLUCOSAMINE'>NAG</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1z8l|1z8l]], [[2c6c|2c6c]], [[2c6p|2c6p]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1z8l|1z8l]], [[2c6c|2c6c]], [[2c6p|2c6p]]</td></tr> | ||
<tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Glutamate_carboxypeptidase_II Glutamate carboxypeptidase II], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.4.17.21 3.4.17.21] </span></td></tr> | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Glutamate_carboxypeptidase_II Glutamate carboxypeptidase II], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.4.17.21 3.4.17.21] </span></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2c6g FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2c6g OCA], [http://pdbe.org/2c6g PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=2c6g RCSB], [http://www.ebi.ac.uk/pdbsum/2c6g PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2c6g FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2c6g OCA], [http://pdbe.org/2c6g PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=2c6g RCSB], [http://www.ebi.ac.uk/pdbsum/2c6g PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=2c6g ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 14: | Line 15: | ||
Check<jmol> | Check<jmol> | ||
<jmolCheckbox> | <jmolCheckbox> | ||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/c6/2c6g_consurf.spt"</scriptWhenChecked> | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/c6/2c6g_consurf.spt"</scriptWhenChecked> | ||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
| Line 46: | Line 47: | ||
[[Category: Slusher, B S]] | [[Category: Slusher, B S]] | ||
[[Category: Tsukamoto, T]] | [[Category: Tsukamoto, T]] | ||
[[Category: Alternative splicing]] | |||
[[Category: Antigen]] | [[Category: Antigen]] | ||
[[Category: Carboxypeptidase]] | [[Category: Carboxypeptidase]] | ||
| Line 57: | Line 59: | ||
[[Category: Neurodegenerative disease]] | [[Category: Neurodegenerative disease]] | ||
[[Category: Peptidase]] | [[Category: Peptidase]] | ||
[[Category: Polymorphism]] | |||
[[Category: Prostate cancer]] | [[Category: Prostate cancer]] | ||
[[Category: Psma]] | [[Category: Psma]] | ||
[[Category: Signal-anchor]] | [[Category: Signal-anchor]] | ||
[[Category: Transmembrane]] | [[Category: Transmembrane]] | ||
[[Category: Zinc]] | |||
Revision as of 11:31, 23 May 2018
membrane-bound glutamate carboxypeptidase II (GCPII) with bound glutamatemembrane-bound glutamate carboxypeptidase II (GCPII) with bound glutamate
Structural highlights
Function[FOLH1_HUMAN] Has both folate hydrolase and N-acetylated-alpha-linked-acidic dipeptidase (NAALADase) activity. Has a preference for tri-alpha-glutamate peptides. In the intestine, required for the uptake of folate. In the brain, modulates excitatory neurotransmission through the hydrolysis of the neuropeptide, N-aceylaspartylglutamate (NAAG), thereby releasing glutamate. Isoform PSM-4 and isoform PSM-5 would appear to be physiologically irrelevant. Involved in prostate tumor progression. Also exhibits a dipeptidyl-peptidase IV type activity. In vitro, cleaves Gly-Pro-AMC. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedMembrane-bound glutamate carboxypeptidase II (GCPII) is a zinc metalloenzyme that catalyzes the hydrolysis of the neurotransmitter N-acetyl-L-aspartyl-L-glutamate (NAAG) to N-acetyl-L-aspartate and L-glutamate (which is itself a neurotransmitter). Potent and selective GCPII inhibitors have been shown to decrease brain glutamate and provide neuroprotection in preclinical models of stroke, amyotrophic lateral sclerosis, and neuropathic pain. Here, we report crystal structures of the extracellular part of GCPII in complex with both potent and weak inhibitors and with glutamate, the product of the enzyme's hydrolysis reaction, at 2.0, 2.4, and 2.2 A resolution, respectively. GCPII folds into three domains: protease-like, apical, and C-terminal. All three participate in substrate binding, with two of them directly involved in C-terminal glutamate recognition. One of the carbohydrate moieties of the enzyme is essential for homodimer formation of GCPII. The three-dimensional structures presented here reveal an induced-fit substrate-binding mode of this key enzyme and provide essential information for the design of GCPII inhibitors useful in the treatment of neuronal diseases and prostate cancer. Structure of glutamate carboxypeptidase II, a drug target in neuronal damage and prostate cancer.,Mesters JR, Barinka C, Li W, Tsukamoto T, Majer P, Slusher BS, Konvalinka J, Hilgenfeld R EMBO J. 2006 Mar 22;25(6):1375-84. Epub 2006 Feb 9. PMID:16467855[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||||
Proteopedia Page Contributors and Editors (what is this?)Proteopedia Page Contributors and Editors (what is this?)
OCA- Glutamate carboxypeptidase II
- Human
- Barinka, C
- Hilgenfeld, R
- Konvalinka, J
- Li, W
- Majer, P
- Mesters, J R
- Slusher, B S
- Tsukamoto, T
- Alternative splicing
- Antigen
- Carboxypeptidase
- Dipeptidase
- Glycoprotein
- Hydrolase
- Metal-binding
- Metalloprotease
- Multifunctional enzyme
- Naaladase
- Neurodegenerative disease
- Peptidase
- Polymorphism
- Prostate cancer
- Psma
- Signal-anchor
- Transmembrane
- Zinc