2vgb: Difference between revisions
No edit summary |
No edit summary |
||
| Line 17: | Line 17: | ||
Check<jmol> | Check<jmol> | ||
<jmolCheckbox> | <jmolCheckbox> | ||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/vg/2vgb_consurf.spt"</scriptWhenChecked> | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/vg/2vgb_consurf.spt"</scriptWhenChecked> | ||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
Revision as of 08:39, 16 May 2018
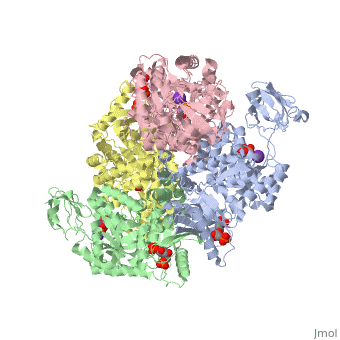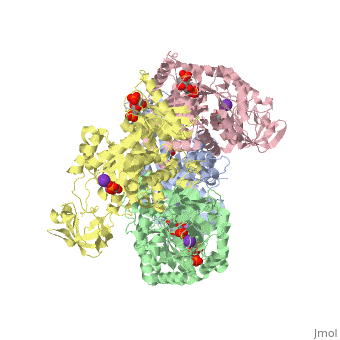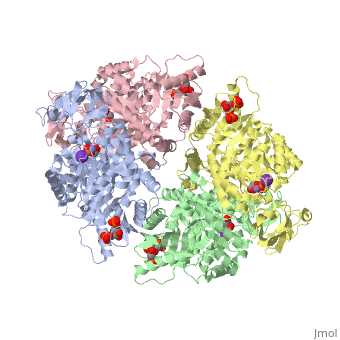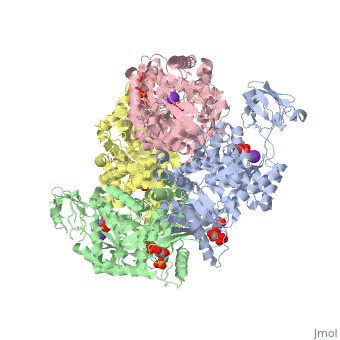
HUMAN ERYTHROCYTE PYRUVATE KINASEHUMAN ERYTHROCYTE PYRUVATE KINASE
Structural highlights
Disease[KPYR_HUMAN] Defects in PKLR are the cause of pyruvate kinase hyperactivity (PKHYP) [MIM:102900]; also known as high red cell ATP syndrome. This autosomal dominant phenotype is characterized by increase of red blood cell ATP.[1] Defects in PKLR are the cause of pyruvate kinase deficiency of red cells (PKRD) [MIM:266200]. A frequent cause of hereditary non-spherocytic hemolytic anemia. Clinically, pyruvate kinase-deficient patients suffer from a highly variable degree of chronic hemolysis, ranging from severe neonatal jaundice and fatal anemia at birth, severe transfusion-dependent chronic hemolysis, moderate hemolysis with exacerbation during infection, to a fully compensated hemolysis without apparent anemia. Function[KPYR_HUMAN] Plays a key role in glycolysis (By similarity). Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedDeficiency of human erythrocyte isozyme (RPK) is, together with glucose-6-phosphate dehydrogenase deficiency, the most common cause of the nonspherocytic hemolytic anemia. To provide a molecular framework to the disease, we have solved the 2.7 A resolution crystal structure of human RPK in complex with fructose 1,6-bisphosphate, the allosteric activator, and phosphoglycolate, a substrate analogue, and we have functionally and structurally characterized eight mutants (G332S, G364D, T384M, D390N, R479H, R486W, R504L, and R532W) found in RPK-deficient patients. The mutations target distinct regions of RPK structure, including domain interfaces and catalytic and allosteric sites. The mutations affect to a different extent thermostability, catalytic efficiency, and regulatory properties. These studies are the first to correlate the clinical symptoms with the molecular properties of the mutant enzymes. Mutations greatly impairing thermostability and/or activity are associated with severe anemia. Some mutant proteins exhibit moderate changes in the kinetic parameters, which are sufficient to cause mild to severe anemia, underlining the crucial role of RPK for erythrocyte metabolism. Prediction of the effects of mutations is difficult because there is no relation between the nature and location of the replaced amino acid and the type of molecular perturbation. Characterization of mutant proteins may serve as a valuable tool to assist with diagnosis and genetic counseling. Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia.,Valentini G, Chiarelli LR, Fortin R, Dolzan M, Galizzi A, Abraham DJ, Wang C, Bianchi P, Zanella A, Mattevi A J Biol Chem. 2002 Jun 28;277(26):23807-14. Epub 2002 Apr 17. PMID:11960989[2] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||