|
|
| Line 1: |
Line 1: |
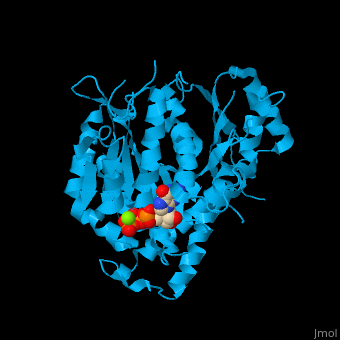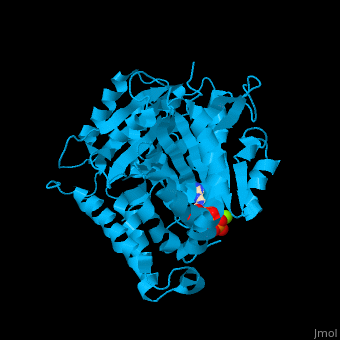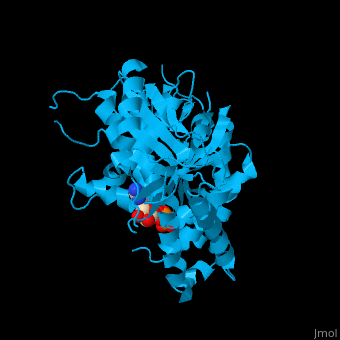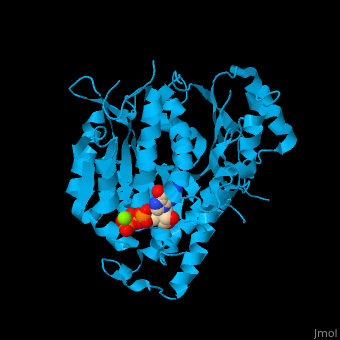
| <StructureSection load='1z5w' size='400' side='right' caption='γ-tubulin complex with GTP and Mg+2 ion (green), [[1z5w]]' scene='43/430888/Cv/2' pspeed='8'> | | <StructureSection load='' size='400' side='right' caption='γ-tubulin complex with GTP and Mg+2 ion (green), [[1z5w]]' scene='43/430888/Cv/2' pspeed='8'> |
| == Function == | | == Function == |
|
| |
|
Revision as of 07:12, 16 October 2017
FunctionTubulin (TUB) is a constituent of microtubules in eukaryotes. Microtubules are assembled from dimers of α-tubulin (TUBA) and β-tubulin (TUBB) bound to GTP. See also Beta tubulin. γ-tubulin (TUBG) is part of the nucleation of microtubules[1]. Human TUB contains several numbered subtypes. FtsZ (Filamenting temperature-sensitive mutant Z) is a prokaryotic homologue of TUB[2]. See also Beta tubulin.
RelevanceThe chemotherapeutic drug Taxol interacts with TUBB. See Molecular Playground/Taxol. TUBA and TUBB expression in polyps of invasive colon cancer is different from normal[3].
DiseaseMutations in TUBB underlie a large spectrum of neuronal migration disorders[4].
Structural highlights | |
3D Structures of Tubulin3D Structures of Tubulin
Updated on 16-October-2017
{"openlevels":0}
- α-tubulin
- 2bto – PdTUBA+thioredoxin 1 – Prosthecobacter dejongeii
- 2e4h – hTUBA C terminal + restin CAP-Gly domain 2 – human
- α-tubulin + β-tubulin
- 1jff - bTUBA+bTUBB - bovine
- 3j6f - pTUBA+pTUBB – pig - Cryo EM
- 3j6e - pTUBA+pTUBB +GMPCPP – Cryo EM
- 3j7i - pTUBA+pTUBB +GTP – Cryo EM
- 1tub - pTUBA+pTUBB – electron diffraction
- 2btq – PdTUBA+PdTUBB
- 5w3f - yTUBA+yTUBB + GDP + GTP - yeast
- 5w3j - yTUBA+yTUBB (mutant) + GDP + GTP + taxol
- 5ubq, 5ucy - TUBA+ TUBB – Tetrahymena thermophila - Cryo EM
- α-tubulin + β-tubulin complexes with stathmin
- 3hkb, 3hkc, 3hkd, 3hke, 3n2g, 3n2k, 3ryc, 3ryf, 3ryh, 3ryi, 4eb6 – sTUBA+sTUBB+stathmin-4 – sheep
- 4f61 - sTUBA+sTUBB+stathmin-like protein R4
- 3ut5 - sTUBA+sTUBB+stathmin-4 + vinca peptide
- 4eb6 - sTUBA+sTUBB+stathmin-4
- 4x1i, 4x1k, 4x1y, 4x20 - sTUBA+bTUBB+stathmin-4 + TUB-Tyr ligase + GDP + GTP + dolastatin + colchicine
- 5kx5 - sTUBA+sTUBB+stathmin-4 + TUB-Tyr ligase + GDP + GTP + ADP + inhibitor
- 5eyp - sTUBA+sTUBB+stathmin-4 + TUB-Tyr ligase + GDP + GTP + colchicine
- 4f6r - sTUBA+sTUBB+stathmin-like protein R1 + ankyrin repeat protein
- 5lp6 – bTUBA1B +bTUBB2B +stathmin-4 + protein
- 3du7, 3e22, 1z2b, 1sa0, 1sa1, 1ffx – bTUBA1C+bTUBB2B+stathmin-4
- 4o2a, 4iij, 4i55 - bTUBA+bTUBB+stathmin-4 + TUB-Tyr ligase
- 4wbn - bTUBA+bTUBB+stathmin-4 + TUB-Tyr ligase + GDP + GTP + ATP derivative
- 5jh7 - bTUBA+bTUBB+stathmin-4 + TUB-Tyr ligase + GDP + GTP + ATP derivative + eribulin
- 5la6, 5m7e, 5bmv, 5lov, 5lxt, 5lxj, 5m7e, 5m7g, 5m8d, 5m8g, 5mf4, 5ov7, 5lxs, 5xlt, 5o7a - bTUBA+bTUBB+stathmin-4 + protein + GDP + GTP + ATP derivative + inhibitor
- 5njh - bTUBA+bTUBB+stathmin-4 + protein + GDP + GTP + ATP derivative + triazolopyrimidine
- 5iyz, 5j2t, 5j2u, 4tv9, 4yj2, 4yj3, 2xrp – bTUBAB1 +bTUBBB2 +stathmin-4 + TUB-Tyr ligase + GDP + GTP + ATP derivative + auristatin anti-mitotics
- 4o2b - bTUBA+bTUBB+stathmin-4 + TUB-Tyr ligase + colchicine
- 4tuy, 4o4h, 4o4i, 4o4j, 4o4l, 4i50, 4i4t, 4tv8, 4zhq, 4zi7, 4zol – pTUBAB1 +pTUBBB2 +stathmin-4 + TUB-Tyr ligase + GDP + GTP + ATP derivative + valinamide derivative
- 4ihj - bTUBA+bTUBB+stathmin-4 + tubulin Tyr-ligase + ADP
- α-tubulin + β-tubulin complex with kinesin
- 4lnu - sTUBA+sTUBB + ankyrin repeat protein + GDP + GTP + kinesin heavy chain
- 2wbe – bTUBA1D+bTUBB2B+kinesin+AMPPNP
- 3dco - bTUBA+bTUBB+kinesin-like NOD
- 4ck5, 4ck6, 4ck7 - bTUBA+bTUBB+kinesin-like KIF11 motor domain
- 5mio - bTUBA+bTUBB+kinesin-like KIF2C motor domain
- 5hnw, 5hnx, 5hny, 5hnz – bTUBAB1 +bTUBBB2 +kinesin-14 + GDP + GTP + taxol
- 3edl - bTUBA1D+bTUBB2B+kinesin motor domain
- 4hna - bTUBA+bTUBB + kinesin heavy chain + ankyrin repeat protein
- 4uxo, 4uxp, 4uxr, 4uxs, 4uxy - bTUBA+bTUBB + kinesin motor domain + GDP + GTP + taxol – Cryo EM
- 4uxt, 4uy0 - bTUBA+bTUBB + kinesin heavy chain + GDP + GTP + taxol – Cryo EM
- 3j2u - bTUBA+bTUBB+kinesin-like KLP10A head domain
- 2p4n - hTUBA+hTUBB+kinesin heavy chain
- 2hxf - pTUBA+pTUBB+kinesin-like KIF1A+AMPPNP
- 3j6h - pTUBA+pTUBB +GMPCPP + kinesin heavy chain – Cryo EM
- 3j8x - pTUBA+pTUBB +GDP + GTP + kinesin heavy chain – Cryo EM
- 3j8y - pTUBA+pTUBB +GDP + GTP + ATP + kinesin heavy chain – Cryo EM
- 2hxh - pTUBA+pTUBB+kinesin-like KIF1A+ADP
- 1ia0 - pTUBA+pTUBB+kinesin-like KIF1A+ATP
- 4aqv, 4aqw - bTUBA+bTUBB+kinesin-like KIF11 – Cryo EM
- 5nd3 - bTUBA+bTUBB+kinesin-like KIF20A + GDP + GTP – Cryo EM
- 5nd2, 5nd4 - bTUBA+bTUBB+kinesin-like KIF20A + GDP + GTP + ADP – Cryo EM
- 5nd7 - bTUBA+bTUBB+kinesin-like KIF20A + AMPPNP – Cryo EM
- 4atx - bTUBA1D+bTUBB2B+kinesin motor domain – Cryo EM
- 5m5i, 5m5l, 5m5m, 5m5n, 5m5o - bTUBA1D+bTUBB2B+kinesin-5 motor domain + AMPPNP – Cryo EM
- 5o09 - TUBA+TUBB +GDP + GTP + kinesin light chain – Prosthecobacter dejongeii - Cryo EM
- α-tubulin + β-tubulin other complexes
- 4drx - sTUBA+sTUBB + ankyrin repeat protein
- 1tvk - bTUBA+bTUBB+GDP+GTP+Epothilone A – electron crystallography
- 3iz0 - bTUBA+bTUBB+hNDC80/SPC25+hNUF2/SPC24 – electron microscopy
- 3j1t, 3j1u - bTUBA+bTUBB+ dynein
- 3j6p - pTUBA+pTUBB +GTP + GDP + taxol + dynein heavy chain – Cryo EM
- 3j6g - pTUBA+pTUBB +GTP + GDP + taxol – Cryo EM
- 5kmg - pTUBA+pTUBB + protein regulator + GTP + GDP – Cryo EM
- 4abo - pTUBA+pTUBB+ microtubule integrity protein MAL3
- 4atu - bTUBA1D+bTUBB2B+doublecortin – Cryo EM
- 5nqu, 5nqt - bTUBA+bTUBB + ankyrin repeat protein + GDP + GTP
- 5nm5 - bTUBA+bTUBB + ankyrin repeat protein + GDP + GTP + colchicine
- 5m50, 5m54, 5m5c - bTUBA+bTUBB + calmodulin-regulated spectrin-associated protein + GDP + GTP - Cryo EM
- 4ffb, 4u3j - yTUBA+TUBB (mutant) + protein STU2
- γ-tubulin
- FtsZ
- 2vxy, 2vam – BsFtsZ - Bacillus subtilis
- 2ve8 – PaFtsZ gamma domain – Pseudomonas aeruginosa
- 2r6r– AaFtsZ - Aquifex aeolicus
- 1w59, 1fsz – MjFtsZ - Methancaldococcus jannaschii
- 1w5e, 1w5f – MjFtsZ (mutant)
- 3v3t – FtsZ (mutant) – Clostridium botulinum
- 3zid – FtsZ – Methanosaeta thermophila
- 3vo9, 3vpa - SaFtsZ – Staphylococcus aureus
- 3wgj, 3wgk – SaFtsZ (mutant)
- FtsZ complexes
- 2rhh, 2rhj – BsFtsZ+SO4
- 2rhl - BsFtsZ+GDP
- 2rho - BsFtsZ+GDP+GTP-gamma-S
- 4u39 – BsFtsZ + cell division factor
- 2ve9 - PaFtsZ gamma domain+DNA
- 2vaw – PaFtsZ+GDP
- 1ofu - PaFtsZ+SULA
- 2r75 - AaFtsZ+8-morpholino-GTP
- 2q1x, 1rq2 - MtFtsZ+citrate – Mycobacterium tuberculosis
- 2q1y, 1rlu - MtFtsZ+GTP-gamma-S
- 1rq7, 4kwe, 5v68 - MtFtsZ+GDP
- 2vap – MjFtsZ+GDP
- 1w58 - MjFtsZ+GMPCPP
- 1w5a - MjFtsZ+MgGTP
- 1w5b - MjFtsZ+GTP
- 4b45 – HvFtsZ + GTP-γS – Haloferax volcanii
- 3vo8, 3voa, 5h5g - SaFtsZ + GDP
- 3wgl, 5xdw, 5h5h, 5h5i, 5mn4, 5mn6 – SaFtsZ (mutant) + GDP
- 3wgn – SaFtsZ + GTP-γS
- 3wgm, 5mn5, 5mn7, 5mn8 – SaFtsZ (mutant) + GTP
- 3vob, 5xdt, 5xdu - SaFtsZ + GDP + inhibitor
- 5xdv - SaFtsZ (mutant) + GDP + inhibitor
- 4dxd - SaFtsZ + antibiotic
- 4b46 - HvFtsZ + GDP
- 4m8i - SeFtsZ + GDP – Staphylococcus epidermitis
ReferencesReferences
- ↑ Moritz M, Agard DA. Gamma-tubulin complexes and microtubule nucleation. Curr Opin Struct Biol. 2001 Apr;11(2):174-81. PMID:11297925
- ↑ Arjes HA, Lai B, Emelue E, Steinbach A, Levin PA. Mutations in the bacterial cell division protein FtsZ highlight the role of GTP binding and longitudinal subunit interactions in assembly and function. BMC Microbiol. 2015 Oct 13;15:209. doi: 10.1186/s12866-015-0544-z. PMID:26463348 doi:http://dx.doi.org/10.1186/s12866-015-0544-z
- ↑ Giarnieri E, De Francesco GP, Carico E, Midiri G, Amanti C, Giacomelli L, Tucci G, Gidaro S, Stroppa I, Gidaro G, Giovagnoli MR. Alpha- and beta-tubulin expression in rectal cancer development. Anticancer Res. 2005 Sep-Oct;25(5):3237-41. PMID:16101133
- ↑ Jaglin XH, Poirier K, Saillour Y, Buhler E, Tian G, Bahi-Buisson N, Fallet-Bianco C, Phan-Dinh-Tuy F, Kong XP, Bomont P, Castelnau-Ptakhine L, Odent S, Loget P, Kossorotoff M, Snoeck I, Plessis G, Parent P, Beldjord C, Cardoso C, Represa A, Flint J, Keays DA, Cowan NJ, Chelly J. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009 Jun;41(6):746-52. doi: 10.1038/ng.380. Epub 2009 May 24. PMID:19465910 doi:http://dx.doi.org/10.1038/ng.380