1ya5: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
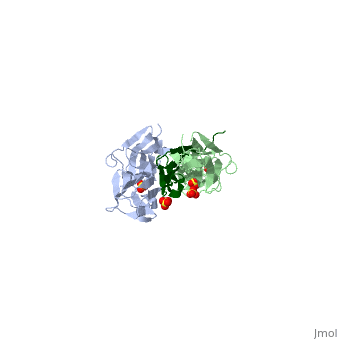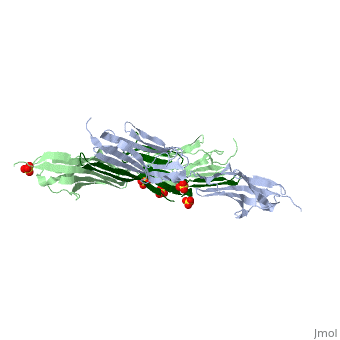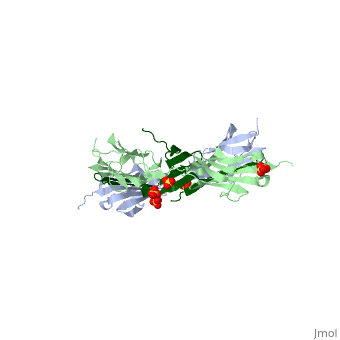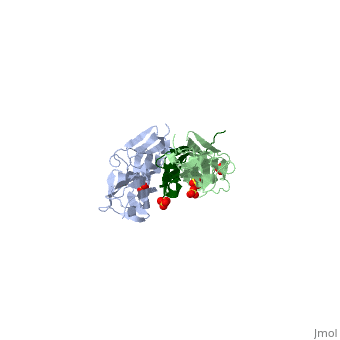
|PDB= 1ya5 |SIZE=350|CAPTION= <scene name='initialview01'>1ya5</scene>, resolution 2.445Å | |PDB= 1ya5 |SIZE=350|CAPTION= <scene name='initialview01'>1ya5</scene>, resolution 2.445Å | ||
|SITE= | |SITE= | ||
|LIGAND= <scene name='pdbligand=SO4:SULFATE ION'>SO4</scene> | |LIGAND= <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene> | ||
|ACTIVITY= | |ACTIVITY= | ||
|GENE= | |GENE= | ||
|DOMAIN= | |||
|RELATEDENTRY= | |||
|RESOURCES=<span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1ya5 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1ya5 OCA], [http://www.ebi.ac.uk/pdbsum/1ya5 PDBsum], [http://www.rcsb.org/pdb/explore.do?structureId=1ya5 RCSB]</span> | |||
}} | }} | ||
| Line 14: | Line 17: | ||
==Overview== | ==Overview== | ||
The Z-disk of striated and cardiac muscle sarcomeres is one of the most densely packed cellular structures in eukaryotic cells. It provides the architectural framework for assembling and anchoring the largest known muscle filament systems by an extensive network of protein-protein interactions, requiring an extraordinary level of mechanical stability. Here we show, using X-ray crystallography, how the amino terminus of the longest filament component, the giant muscle protein titin, is assembled into an antiparallel (2:1) sandwich complex by the Z-disk ligand telethonin. The pseudosymmetric structure of telethonin mediates a unique palindromic arrangement of two titin filaments, a type of molecular assembly previously found only in protein-DNA complexes. We have confirmed its unique architecture in vivo by protein complementation assays, and in vitro by experiments using fluorescence resonance energy transfer. The model proposed may provide a molecular paradigm of how major sarcomeric filaments are crosslinked, anchored and aligned within complex cytoskeletal networks. | The Z-disk of striated and cardiac muscle sarcomeres is one of the most densely packed cellular structures in eukaryotic cells. It provides the architectural framework for assembling and anchoring the largest known muscle filament systems by an extensive network of protein-protein interactions, requiring an extraordinary level of mechanical stability. Here we show, using X-ray crystallography, how the amino terminus of the longest filament component, the giant muscle protein titin, is assembled into an antiparallel (2:1) sandwich complex by the Z-disk ligand telethonin. The pseudosymmetric structure of telethonin mediates a unique palindromic arrangement of two titin filaments, a type of molecular assembly previously found only in protein-DNA complexes. We have confirmed its unique architecture in vivo by protein complementation assays, and in vitro by experiments using fluorescence resonance energy transfer. The model proposed may provide a molecular paradigm of how major sarcomeric filaments are crosslinked, anchored and aligned within complex cytoskeletal networks. | ||
==About this Structure== | ==About this Structure== | ||
| Line 29: | Line 29: | ||
[[Category: Wilmanns, M.]] | [[Category: Wilmanns, M.]] | ||
[[Category: Zou, P.]] | [[Category: Zou, P.]] | ||
[[Category: | [[Category: ig-like domain]] | ||
[[Category: | [[Category: t-cap]] | ||
[[Category: telethonin]] | |||
[[Category: titin]] | |||
[[Category: z1]] | |||
[[Category: z2]] | |||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Mon Mar 31 01:00:45 2008'' | ||
Revision as of 01:00, 31 March 2008
| |||||||
| , resolution 2.445Å | |||||||
|---|---|---|---|---|---|---|---|
| Ligands: | |||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
Crystal structure of the titin domains z1z2 in complex with telethonin
OverviewOverview
The Z-disk of striated and cardiac muscle sarcomeres is one of the most densely packed cellular structures in eukaryotic cells. It provides the architectural framework for assembling and anchoring the largest known muscle filament systems by an extensive network of protein-protein interactions, requiring an extraordinary level of mechanical stability. Here we show, using X-ray crystallography, how the amino terminus of the longest filament component, the giant muscle protein titin, is assembled into an antiparallel (2:1) sandwich complex by the Z-disk ligand telethonin. The pseudosymmetric structure of telethonin mediates a unique palindromic arrangement of two titin filaments, a type of molecular assembly previously found only in protein-DNA complexes. We have confirmed its unique architecture in vivo by protein complementation assays, and in vitro by experiments using fluorescence resonance energy transfer. The model proposed may provide a molecular paradigm of how major sarcomeric filaments are crosslinked, anchored and aligned within complex cytoskeletal networks.
About this StructureAbout this Structure
1YA5 is a Protein complex structure of sequences from Homo sapiens. Full crystallographic information is available from OCA.
ReferenceReference
Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk., Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M, Nature. 2006 Jan 12;439(7073):229-33. PMID:16407954
Page seeded by OCA on Mon Mar 31 01:00:45 2008