1j7n: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Anthrax Toxin Lethal factor== | ==Anthrax Toxin Lethal factor== | ||
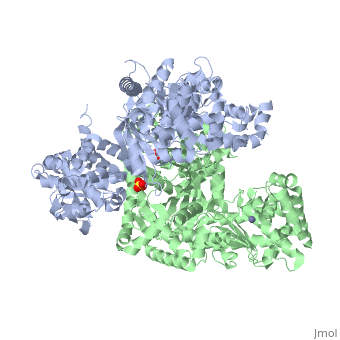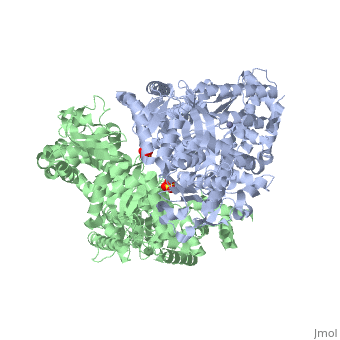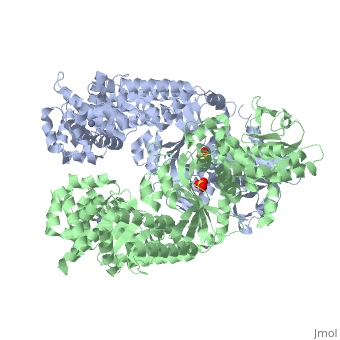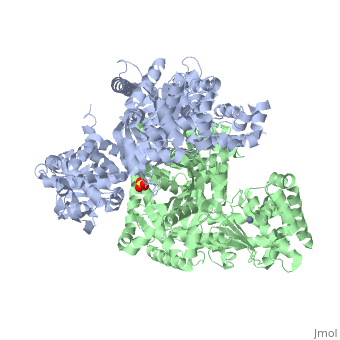
<StructureSection load='1j7n' size='340' side='right' caption='[[1j7n]], [[Resolution|resolution]] 2.30Å' scene=''> | <StructureSection load='1j7n' size='340' side='right' caption='[[1j7n]], [[Resolution|resolution]] 2.30Å' scene=''> | ||
| Line 5: | Line 6: | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">lef ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=1392 "Bacillus cereus var. anthracis" (Cohn 1872) Smith et al. 1946])</td></tr> | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">lef ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=1392 "Bacillus cereus var. anthracis" (Cohn 1872) Smith et al. 1946])</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1j7n FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1j7n OCA], [http://pdbe.org/1j7n PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1j7n RCSB], [http://www.ebi.ac.uk/pdbsum/1j7n PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1j7n FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1j7n OCA], [http://pdbe.org/1j7n PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1j7n RCSB], [http://www.ebi.ac.uk/pdbsum/1j7n PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=1j7n ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
Revision as of 12:51, 4 October 2017
Anthrax Toxin Lethal factorAnthrax Toxin Lethal factor
Structural highlights
Function[LEF_BACAN] One of the three proteins composing the anthrax toxin, the agent which infects many mammalian species and that may cause death. LF is the lethal factor that, when associated with PA, causes death. LF is not toxic by itself. It is a protease that cleaves the N-terminal of most dual specificity mitogen-activated protein kinase kinases (MAPKKs or MAP2Ks) (except for MAP2K5). Cleavage invariably occurs within the N-terminal proline-rich region preceding the kinase domain, thus disrupting a sequence involved in directing specific protein-protein interactions necessary for the assembly of signaling complexes. There may be other cytosolic targets of LF involved in cytotoxicity. The proteasome may mediate a toxic process initiated by LF in the cell cytosol involving degradation of unidentified molecules that are essential for macrophage homeostasis. This is an early step in LeTx intoxication, but it is downstream of the cleavage by LF of MEK1 or other putative substrates.[1] [2] [3] [4] [5] Publication Abstract from PubMedLethal factor (LF) is a protein (relative molecular mass 90,000) that is critical in the pathogenesis of anthrax. It is a highly specific protease that cleaves members of the mitogen-activated protein kinase kinase (MAPKK) family near to their amino termini, leading to the inhibition of one or more signalling pathways. Here we describe the crystal structure of LF and its complex with the N terminus of MAPKK-2. LF comprises four domains: domain I binds the membrane-translocating component of anthrax toxin, the protective antigen (PA); domains II, III and IV together create a long deep groove that holds the 16-residue N-terminal tail of MAPKK-2 before cleavage. Domain II resembles the ADP-ribosylating toxin from Bacillus cereus, but the active site has been mutated and recruited to augment substrate recognition. Domain III is inserted into domain II, and seems to have arisen from a repeated duplication of a structural element of domain II. Domain IV is distantly related to the zinc metalloprotease family, and contains the catalytic centre; it also resembles domain I. The structure thus reveals a protein that has evolved through a process of gene duplication, mutation and fusion, into an enzyme with high and unusual specificity. Crystal structure of the anthrax lethal factor.,Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, Lacy DB, Collier RJ, Park S, Leppla SH, Hanna P, Liddington RC Nature. 2001 Nov 8;414(6860):229-33. PMID:11700563[6] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||