Carbidopa: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
== Molecular Mechanism == | == Molecular Mechanism == | ||
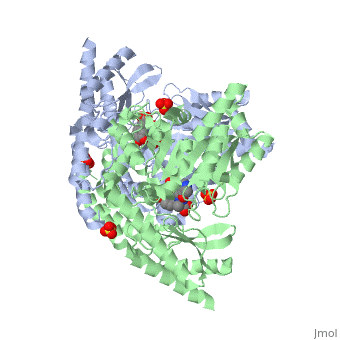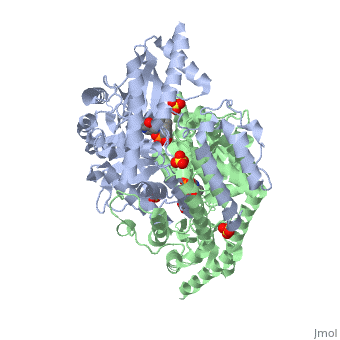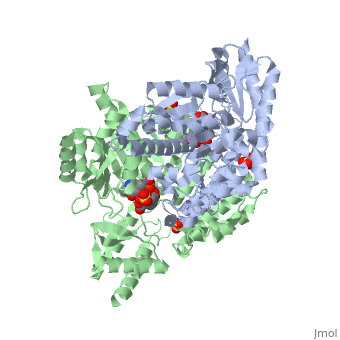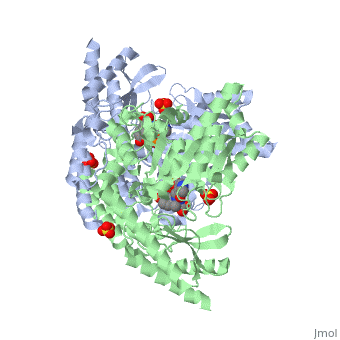
The | The vitamin B6 (<scene name='pdbligand=PLP:PYRIDOXAL-5-PHOSPHATE'>PLP</scene>)-dependent enzyme DDC, an enzyme abundant in the nervous system as well as kidneys of humans, catalyzes the conversion of L-DOPA into dopamine.<ref name="three">PMID: 7651438</ref> The catalytically active form of DDC is a homodimer, a feature typical of this class of enzymes.<ref name= "four">PMID: 10673430</ref> DDCs active site is located between the two monomers but is mainly composed of residues from only one of the monomers. The cofactor PLP binds to Lys 303 through a Schiff base linkage and a salt bridge between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP, which acts as a strong electron sink capable of stabilizing the carbanionic intermediates produced by active DDC. the cofactor is further stabilized in the enzyme through a network of hydrogen bonds. Carbidopa works by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety and blocking the DDC <scene name='74/746001/Active_site_dopa_final/1'>active site</scene> residues Ile 101' and Phe 103' in the substrate binding pocket with its catechol ring.<ref name="five">doi:10.1038/nsb1101-963</ref> In addition, the 4' hydroxyl group of Carbidopas catechol ring hydrogen bonds with THR 82 and the carboxylate group binds to HIS 192, a highly-conserved residue in PLP-dependent decarboxylases.<ref name="six">PMID: 8889823</ref> Due to the fact that Carbidopa cannot cross the blood-brain barrier, its inhibiting effects only are displayed in the periphery. | ||
==Interactions== | ==Interactions== | ||
Revision as of 06:45, 17 November 2016
Carbidoba ((2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid)Carbidoba ((2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid)
Function(Lodosyn) is a drug given, in conjunction with Levadopa, to those with Parkinson's Disease in order to inhibit the extracranial metabolism of Levadopa to dopamine, allowing a greater portion of the drug to cross the blood-brain barrier and produce the desired anti-parkinsonian effects in the nerve cell. It works by inhibiting the enzymatic activity of aromatic-L-amino-acid decarboxylase (DOPA Decarboxylase or DDC).[1] Structure  Carbidopa is an inhibitor of DDC. It is a partially water soluble, white, crystalline compound with a molecular weight of 226.232g/mol and a melting point of 208°C. Its empirical formula is C10H14N2O4•H2O and its IUPAC name is (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid. Used in conjunction with Levadopa (L-DOPA), a precursor to dopamine, it increases concentrations of L-DOPA in the brain. Due to the fact Carbidopa cannot cross the blood–brain barrier, it inhibits only peripheral DDC thus preventing the conversion of L-DOPA to dopamine outside of neuronal cells. This greatly lessens the side effects caused by dopamine on the periphery, as well as increasing the concentration of L-DOPA and subsequently dopamine in the brain.[2] Molecular MechanismThe vitamin B6 ()-dependent enzyme DDC, an enzyme abundant in the nervous system as well as kidneys of humans, catalyzes the conversion of L-DOPA into dopamine.[3] The catalytically active form of DDC is a homodimer, a feature typical of this class of enzymes.[4] DDCs active site is located between the two monomers but is mainly composed of residues from only one of the monomers. The cofactor PLP binds to Lys 303 through a Schiff base linkage and a salt bridge between the carboxylate group of Asp 271 and the protonated pyridine nitrogen of PLP, which acts as a strong electron sink capable of stabilizing the carbanionic intermediates produced by active DDC. the cofactor is further stabilized in the enzyme through a network of hydrogen bonds. Carbidopa works by forming a hydrazone linkage with the PLP cofactor through its hydrazine moiety and blocking the DDC residues Ile 101' and Phe 103' in the substrate binding pocket with its catechol ring.[5] In addition, the 4' hydroxyl group of Carbidopas catechol ring hydrogen bonds with THR 82 and the carboxylate group binds to HIS 192, a highly-conserved residue in PLP-dependent decarboxylases.[6] Due to the fact that Carbidopa cannot cross the blood-brain barrier, its inhibiting effects only are displayed in the periphery. Interactions  Carbidopa only has interactions with Levodopa (L-DOPA) and dopamine, but there are more interactions between Levodopa and dopamine. Dopamine is essential when it comes to motor, cognitive, behavioral, endocrine functions, and even neuronal retinal development in the central nervous system.[7][8] Therefore,understanding the interactions between how Levodopa and dopamine affect the body can help understand why it pairs well with Carbidopa. Carbidopa works by inhibiting the enzyme activity of DDC, when both are working in the body it blocks the Levodopa negative side effects so the lipid soluble drug pair can now pass the blood-brain barrier where Carbidopa cannot. Carbidopa only interaction is to reduce side effects of Levodopa because once Levodopa crosses the blood brain barrier it can help increase levels of dopamine to improve motor, cognitive, behavioral, endocrine functions, etc. Another likely interaction between dopamine and Carbidopa is the human peripheral blood lymphocytes. These blood lymphocytes supposedly synthesize dopamine through the tyrosine-hydroxylase/DOPA-decarboxylase pathway, and express dopamine receptors and dopamine transport on their plasma membrane.[8] Reports show changes in expression of this dopamine receptors and dopamine transport system in the peripheral blood lymphocytes, but since the Carbidopa can’t pass the blood brain barrier it needs to be paired to a lipid soluble chemical (Levodopa) until a different method is found. Disease in HumansParkinson's disease (PD) is a chronic, progressive neurological disease whose symptoms include bradykinesia, tremors, postural instability and rigidity. Although the exact cause of the disease is currently unknown, it is believed to be caused by the apoptosis of dopaminergic cells in the substantia nigra of the brain and subsequent loss of dopamine.[9] Carbidopa is mostly related to people with Parkinson’s disease. According to the National Parkinson Foundation, Levodopa alone is known to cause nausea and vomiting in Parkinson’s patients, and Carbidopa prevents those side effects.[10] Carbidopa can act as an enhancer for Levodopa by decreasing the dosage of Levodopa needed for Parkinson’s patients, up to 80%. It’s a inhibitor that is limited to extracerebral tissue, therefore Carbidopa with Levodopa leaves more Levodopa available for transport to the brain.[11] Modern treatments like this are used for Parkinson’s disease today. A combined tablet of Carbidopa and Levodopa (Sinemet)are offered as immediate-release tablets and slow-release tablets, along with dissolvable tablets.[12]
|
| ||||||||||
ReferencesReferences
- ↑ Gilbert JA, Frederick LM, Ames MM. The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res. 2000 Nov;6(11):4365-72. PMID:11106255
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/carbidopa
- ↑ Opacka-Juffry J, Brooks DJ. L-dihydroxyphenylalanine and its decarboxylase: new ideas on their neuroregulatory roles. Mov Disord. 1995 May;10(3):241-9. PMID:7651438 doi:http://dx.doi.org/10.1002/mds.870100302
- ↑ Schneider G, Kack H, Lindqvist Y. The manifold of vitamin B6 dependent enzymes. Structure. 2000 Jan 15;8(1):R1-6. PMID:10673430
- ↑ Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN. Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase. Nat Struct Biol. 2001 Nov;8(11):963-7. PMID:11685243 doi:http://dx.doi.org/10.1038/nsb1101-963
- ↑ Ishii S, Mizuguchi H, Nishino J, Hayashi H, Kagamiyama H. Functionally important residues of aromatic L-amino acid decarboxylase probed by sequence alignment and site-directed mutagenesis. J Biochem. 1996 Aug;120(2):369-76. PMID:8889823
- ↑ Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008 Sep 30;6(9):e236. doi: 10.1371/journal.pbio.0060236. PMID:18828673 doi:http://dx.doi.org/10.1371/journal.pbio.0060236
- ↑ 8.0 8.1 Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE. The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Curr Neuropharmacol. 2011 Jun;9(2):278-88. doi: 10.2174/157015911795596612. PMID:22131937 doi:http://dx.doi.org/10.2174/157015911795596612
- ↑ Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000 Mar 23;404(6776):394-8. PMID:10746727 doi:http://dx.doi.org/10.1038/35006074
- ↑ http://www.parkinson.org/understanding-parkinsons/treatment/Medications-for-Motor-Symptoms/Carbidopa-levodopa
- ↑ Fanali S, Pucci V, Sabbioni C, Raggi MA. Quality control of benserazide-levodopa and carbidopa-levodopa tablets by capillary zone electrophoresis. Electrophoresis. 2000 Jul;21(12):2432-7. PMID:10939456 doi:<2432::AID-ELPS2432>3.0.CO;2-E http://dx.doi.org/10.1002/1522-2683(20000701)21:12<2432::AID-ELPS2432>3.0.CO;2-E
- ↑ http://www.merck.com/product/home.html