4adl: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
==Crystal structures of Rv1098c in complex with malate== | ==Crystal structures of Rv1098c in complex with malate== | ||
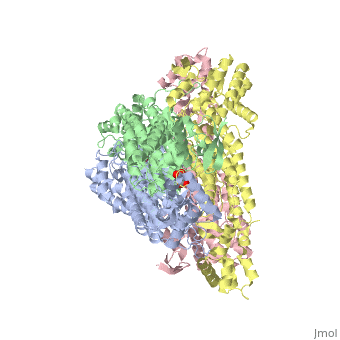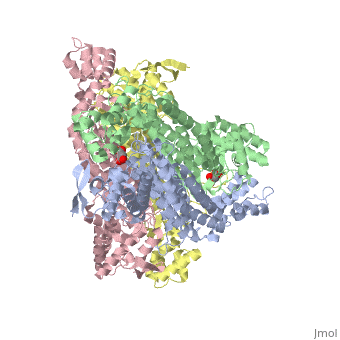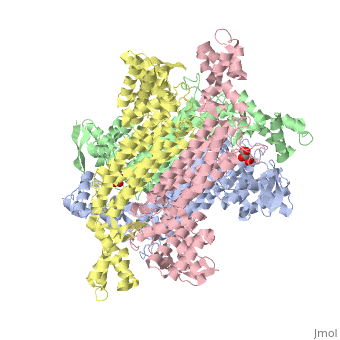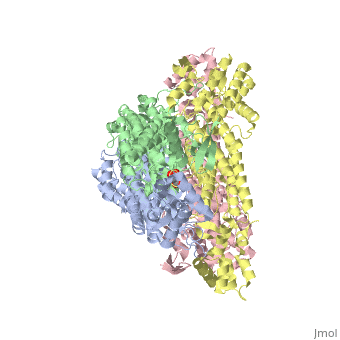
<StructureSection load='4adl' size='340' side='right' caption='[[4adl]], [[Resolution|resolution]] 2.20Å' scene=''> | <StructureSection load='4adl' size='340' side='right' caption='[[4adl]], [[Resolution|resolution]] 2.20Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[4adl]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[4adl]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Myctu Myctu]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4ADL OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4ADL FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=LMR:(2S)-2-HYDROXYBUTANEDIOIC+ACID'>LMR</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=LMR:(2S)-2-HYDROXYBUTANEDIOIC+ACID'>LMR</scene></td></tr> | ||
<tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[4adm|4adm]], [[4apa|4apa]], [[4apb|4apb]]</td></tr> | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[4adm|4adm]], [[4apa|4apa]], [[4apb|4apb]]</td></tr> | ||
<tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Fumarate_hydratase Fumarate hydratase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.2.1.2 4.2.1.2] </span></td></tr> | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/Fumarate_hydratase Fumarate hydratase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.2.1.2 4.2.1.2] </span></td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4adl FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4adl OCA], [http://www.rcsb.org/pdb/explore.do?structureId=4adl RCSB], [http://www.ebi.ac.uk/pdbsum/4adl PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4adl FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4adl OCA], [http://pdbe.org/4adl PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=4adl RCSB], [http://www.ebi.ac.uk/pdbsum/4adl PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=4adl ProSAT]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 18: | Line 19: | ||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
</div> | </div> | ||
<div class="pdbe-citations 4adl" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
| Line 26: | Line 28: | ||
</StructureSection> | </StructureSection> | ||
[[Category: Fumarate hydratase]] | [[Category: Fumarate hydratase]] | ||
[[Category: | [[Category: Myctu]] | ||
[[Category: Alzari, P M]] | [[Category: Alzari, P M]] | ||
[[Category: Bellinzoni, M]] | [[Category: Bellinzoni, M]] | ||
Revision as of 16:41, 5 August 2016
Crystal structures of Rv1098c in complex with malateCrystal structures of Rv1098c in complex with malate
Structural highlights
Function[FUMC_MYCTU] Catalyzes the reversible addition of water to fumarate to give L-malate. Publication Abstract from PubMedrv1098c, an essential gene in Mycobacterium tuberculosis, codes for a class II fumarase. We describe here the crystal structure of Rv1098c in complex with l-malate, fumarate or the competitive inhibitor meso-tartrate. The models reveal that substrate binding promotes the closure of the active site through conformational changes involving the catalytic SS-loop and the C-terminal domain, which likely represents a general feature of this enzyme superfamily. Analysis of ligand-enzyme interactions as well as site-directed mutagenesis suggest Ser318 as one of the two acid-base catalysts. STRUCTURED SUMMARY OF PROTEIN INTERACTIONS: Rv1098c and Rv1098c bind by X-ray crystallography (View interaction). Conformational changes upon ligand binding in the essential class II fumarase Rv1098c from Mycobacterium tuberculosis.,Mechaly AE, Haouz A, Miras I, Barilone N, Weber P, Shepard W, Alzari PM, Bellinzoni M FEBS Lett. 2012 Jun 4;586(11):1606-11. Epub 2012 May 3. PMID:22561013[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||