7cei: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
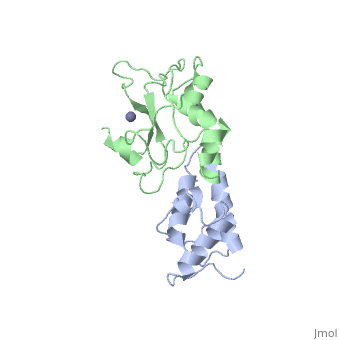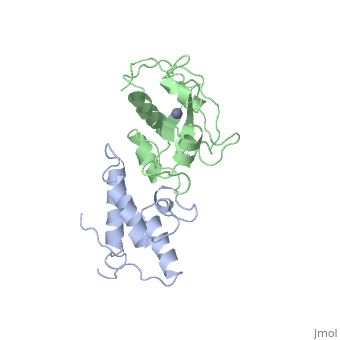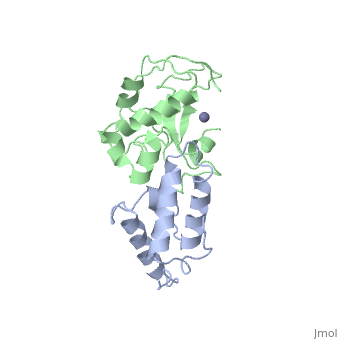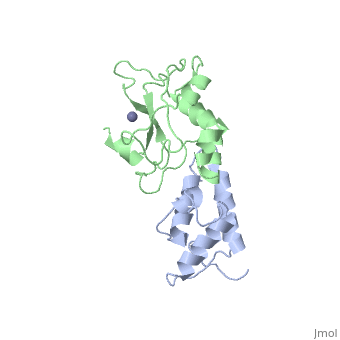
<StructureSection load='7cei' size='340' side='right' caption='[[7cei]], [[Resolution|resolution]] 2.30Å' scene=''> | <StructureSection load='7cei' size='340' side='right' caption='[[7cei]], [[Resolution|resolution]] 2.30Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[7cei]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[7cei]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Ecow3 Ecow3]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=7CEI OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=7CEI FirstGlance]. <br> | ||
</td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | ||
<tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">CEIE7 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=316407 | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">CEIE7 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=316407 ECOW3])</td></tr> | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=7cei FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=7cei OCA], [http://www.rcsb.org/pdb/explore.do?structureId=7cei RCSB], [http://www.ebi.ac.uk/pdbsum/7cei PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=7cei FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=7cei OCA], [http://pdbe.org/7cei PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=7cei RCSB], [http://www.ebi.ac.uk/pdbsum/7cei PDBsum]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
[[http://www.uniprot.org/uniprot/ | [[http://www.uniprot.org/uniprot/IMM7_ECOLX IMM7_ECOLX]] This protein is able to protect a cell, which harbors the plasmid ColE7 encoding colicin E7, against colicin E7, it binds specifically to the DNase-type colicin and inhibits its bactericidal activity. Dimeric ImmE7 may possess a RNase activity that cleaves its own mRNA at a specific site and thus autoregulates translational expression of the downstream ceiE7 gene as well as degradation of the upstream ceaE7 mRNA. [[http://www.uniprot.org/uniprot/CEA7_ECOLX CEA7_ECOLX]] This plasmid-coded bactericidal protein is an endonuclease active on both single- and double-stranded DNA but with undefined specificity. Colicins are polypeptide toxins produced by and active against E.coli and closely related bacteria. | ||
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 17: | Line 17: | ||
<text>to colour the structure by Evolutionary Conservation</text> | <text>to colour the structure by Evolutionary Conservation</text> | ||
</jmolCheckbox> | </jmolCheckbox> | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/ | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=7cei ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
| Line 27: | Line 27: | ||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
</div> | </div> | ||
<div class="pdbe-citations 7cei" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
| Line 34: | Line 35: | ||
*[[Colicin Immunity Protein 9|Colicin Immunity Protein 9]] | *[[Colicin Immunity Protein 9|Colicin Immunity Protein 9]] | ||
*[[Directed evolution|Directed evolution]] | *[[Directed evolution|Directed evolution]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Ecow3]] | ||
[[Category: Chak, K F]] | [[Category: Chak, K F]] | ||
[[Category: Ko, T P]] | [[Category: Ko, T P]] | ||
Revision as of 04:49, 10 February 2016
THE ENDONUCLEASE DOMAIN OF COLICIN E7 IN COMPLEX WITH ITS INHIBITOR IM7 PROTEINTHE ENDONUCLEASE DOMAIN OF COLICIN E7 IN COMPLEX WITH ITS INHIBITOR IM7 PROTEIN
Structural highlights
Function[IMM7_ECOLX] This protein is able to protect a cell, which harbors the plasmid ColE7 encoding colicin E7, against colicin E7, it binds specifically to the DNase-type colicin and inhibits its bactericidal activity. Dimeric ImmE7 may possess a RNase activity that cleaves its own mRNA at a specific site and thus autoregulates translational expression of the downstream ceiE7 gene as well as degradation of the upstream ceaE7 mRNA. [CEA7_ECOLX] This plasmid-coded bactericidal protein is an endonuclease active on both single- and double-stranded DNA but with undefined specificity. Colicins are polypeptide toxins produced by and active against E.coli and closely related bacteria. Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedBACKGROUND: Colicin E7 (ColE7) is one of the bacterial toxins classified as a DNase-type E-group colicin. The cytotoxic activity of a colicin in a colicin-producing cell can be counteracted by binding of the colicin to a highly specific immunity protein. This biological event is a good model system for the investigation of protein recognition. RESULTS: The crystal structure of a one-to-one complex between the DNase domain of colicin E7 and its cognate immunity protein Im7 has been determined at 2.3 A resolution. Im7 in the complex is a varied four-helix bundle that is identical to the structure previously determined for uncomplexed Im7. The structure of the DNase domain of ColE7 displays a novel alpha/beta fold and contains a Zn2+ ion bound to three histidine residues and one water molecule in a distorted tetrahedron geometry. Im7 has a V-shaped structure, extending two arms to clamp the DNase domain of ColE7. One arm (alpha1(*)-loop12-alpha2(*); where * represents helices in Im7) is located in the region that displays the greatest sequence variation among members of the immunity proteins in the same subfamily. This arm mainly uses acidic sidechains to interact with the basic sidechains in the DNase domain of ColE7. The other arm (loop 23-alpha3(*)-loop 34) is more conserved and it interacts not only with the sidechain but also with the mainchain atoms of the DNase domain of ColE7. CONCLUSIONS: The protein interfaces between the DNase domain of ColE7 and Im7 are charge-complementary and charge interactions contribute significantly to the tight and specific binding between the two proteins. The more variable arm in Im7 dominates the binding specificity of the immunity protein to its cognate colicin. Biological and structural data suggest that the DNase active site for ColE7 is probably near the metal-binding site. The crystal structure of the DNase domain of colicin E7 in complex with its inhibitor Im7 protein.,Ko TP, Liao CC, Ku WY, Chak KF, Yuan HS Structure. 1999 Jan 15;7(1):91-102. PMID:10368275[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See Also
References |
| ||||||||||||||||||