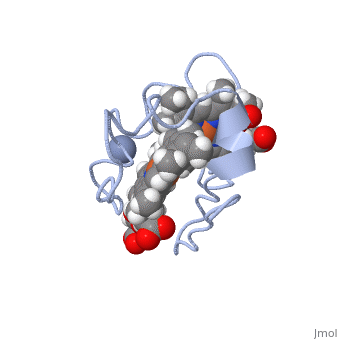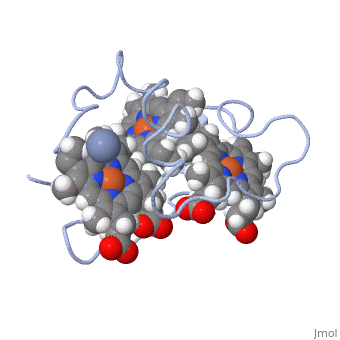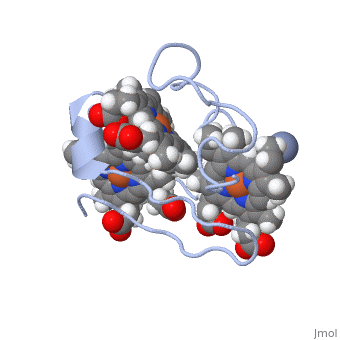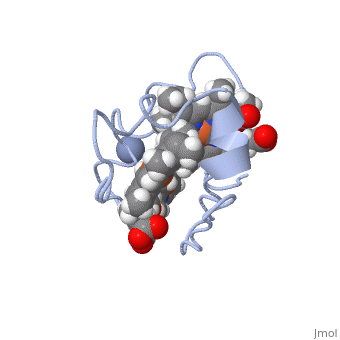Cytochrome c 7: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
<StructureSection load='1LM2.pdb' size='340' side='right' caption='3D Structure of Cytochrome c 7' scene=''> | <StructureSection load='1LM2.pdb' size='340' side='right' caption='3D Structure of Cytochrome c 7' scene=''> | ||
'''Cytocrhome c 7''' (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium ''Desulfuromonas acetoxidans''. Cc7 is | '''Cytocrhome c 7''' (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium ''Desulfuromonas acetoxidans''. Cc7 is crucial to the bacteria's anaerobic sulfure respiration as it plays a role in the electron-transfer mechanism, and as such it is located in the mitochondrial intermembrane space<ref>Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 '''[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC125002/''' DOI: 10.1073/pnas.152290999''']'''</ref>. Over the course of 3.5 million years, ''Desulfuromonas acetoxidans'' have evolved to use anaerobic sulfure respiration as the main driving force of their survival<ref>DOI: 10.1016/s0065-2164(09)01202-7</ref>. As a result, Cc7 is able to reduce sulfure, its oxidized variants (thiosulfate, sulfur, sulfite, sulfate, etc) and even heavy metals to produce the required energy<ref>Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 '''[http://link.springer.com/article/10.1007/BF00416962''' DOI: 10.1007/BF00416962''']'''</ref>. If using sulfure, the product formed is hydrogen sulfide. This compound is able to react with heavy metal ions to form almost non-lethal and quite insoluble metal sulfides<ref>Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 '''[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC125002/''' DOI: 10.1073/pnas.152290999''']'''</ref>. This enzymatic ability of Cc7 is unique to the animal kingdom, and the ability to create less-toxic and insoluble metal sulfides has intrigued researchers as of late<ref>Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 '''[http://link.springer.com/article/10.1007/BF00416962''' DOI: 10.1007/BF00416962''']'''</ref>. By harnessing ''Desulfuromonas acetoxidans'' and its Cc7 protein, researchers hope to create possible applications to use these bacteria to decontaminate environments polluted with toxic heavy metals from industrial wastes across the globe. And because of the metal sulfides insolubility, removing them from the environment will be simpler and cheaper than current heavy metal toxic waste filtration operations<ref>National Service Center for Environmental Publications. [http://nepis.epa.gov/Exe/ZyNET.exe/91018HWZ.txt?ZyActionD=ZyDocument&Client=EPA&Index=1976%20Thru%201980&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A\ZYFILES\INDEX%20DATA\76THRU80\TXT\00000025\91018HWZ.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h|-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=p|f&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1]</ref>. | ||
==Structural Components== | ==Structural Components== | ||
| Line 8: | Line 8: | ||
== Function == | == Function == | ||
Cc7 is a sulfur terminal reductase, that is, it reduces a sulfur-containing compound into a sulfide to generate electrons to be used in electron transport chain. | Cc7 is a sulfur terminal reductase, that is, it reduces a sulfur-containing compound into a sulfide to generate electrons to be used in electron transport chain. The steps required to for this to occur are as follows. | ||
First, Cc7 must be in its resting state. Here, Cc7 is fully reduced and the chromium (III) ion is reduced to chromate(-2). | |||
The products of the reaction are chromium(III) and the oxidized protein with three iron(III) hemes. | |||
== Structural highlights == | == Structural highlights == | ||
Revision as of 09:09, 30 November 2015
GeneralGeneral
Cytocrhome c 7 (Cc7) is a three heme-containing protein derived from the sulfur-reducing bacterium Desulfuromonas acetoxidans. Cc7 is crucial to the bacteria's anaerobic sulfure respiration as it plays a role in the electron-transfer mechanism, and as such it is located in the mitochondrial intermembrane space[1]. Over the course of 3.5 million years, Desulfuromonas acetoxidans have evolved to use anaerobic sulfure respiration as the main driving force of their survival[2]. As a result, Cc7 is able to reduce sulfure, its oxidized variants (thiosulfate, sulfur, sulfite, sulfate, etc) and even heavy metals to produce the required energy[3]. If using sulfure, the product formed is hydrogen sulfide. This compound is able to react with heavy metal ions to form almost non-lethal and quite insoluble metal sulfides[4]. This enzymatic ability of Cc7 is unique to the animal kingdom, and the ability to create less-toxic and insoluble metal sulfides has intrigued researchers as of late[5]. By harnessing Desulfuromonas acetoxidans and its Cc7 protein, researchers hope to create possible applications to use these bacteria to decontaminate environments polluted with toxic heavy metals from industrial wastes across the globe. And because of the metal sulfides insolubility, removing them from the environment will be simpler and cheaper than current heavy metal toxic waste filtration operations[6]. Structural ComponentsCc7 is a single polypeptide chain 68 residues total containing three heme groups. The polypeptide strand has one alpha helix 4 residues in length and two beta strands 2 residues in length. The heme groups are labelled as I, III, and IV, FunctionCc7 is a sulfur terminal reductase, that is, it reduces a sulfur-containing compound into a sulfide to generate electrons to be used in electron transport chain. The steps required to for this to occur are as follows. First, Cc7 must be in its resting state. Here, Cc7 is fully reduced and the chromium (III) ion is reduced to chromate(-2). The products of the reaction are chromium(III) and the oxidized protein with three iron(III) hemes. Structural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 DOI: 10.1073/pnas.152290999
- ↑ DOI: 10.1016/s0065-2164(09)01202-7
- ↑ Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 DOI: 10.1007/BF00416962
- ↑ Assfalg M, Bertini I, Bruschi M, Michel C, Turano P. The metal reductase activity of some multiheme cytochromes c: NMR structural characterization of the reduction of chromium(VI) to chromium(III) by cytochrome c(7). 2002; 99(15):9750-4 DOI: 10.1073/pnas.152290999
- ↑ Pfennig N, Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. 1976; 110(1): 3-12 DOI: 10.1007/BF00416962
- ↑ National Service Center for Environmental Publications. [1]