Cas9 Sandbox: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
== Structural highlights == | == Structural highlights == | ||
Cas9 is a structurally bilobed, containing specific domains for the recognition of target DNA and nucleases to cleave DNA strands. Each of these lobes contain three major units essential for a functional endonuclease, these are the essential <scene name='71/714945/Lobes_domain_of_cas9/ | Cas9 is a structurally bilobed, containing specific domains for the recognition of target DNA and nucleases to cleave DNA strands. Each of these lobes contain three major units essential for a functional endonuclease, these are the essential <scene name='71/714945/Lobes_domain_of_cas9/3'>domains</scene>. | ||
The recognition lobe (REC) contains a long bridge helix, the REC-1 domain and the REC-2 domain. It is also the least conserved lobe through the types of Cas9. The long α-helix bridge, which is arginine-rich, is essential for recognizing single guide RNA-DNA complexes on target DNA. This structure has been shown to be conserved through Cas9 proteins. The REC-1 domain contains 25 α-helixes and two β-sheets, and is crucial to the function of Cas9 by recognizing a specific motif termed the repeat:anti-repeat region of the single guide RNA and DNA complex. The REC-2 domain contains six α-helix’s in a bundle but there is no current understanding of its function. | The recognition lobe (REC) contains a long bridge helix, the REC-1 domain and the REC-2 domain. It is also the least conserved lobe through the types of Cas9. The long α-helix bridge, which is arginine-rich, is essential for recognizing single guide RNA-DNA complexes on target DNA. This structure has been shown to be conserved through Cas9 proteins. The REC-1 domain contains 25 α-helixes and two β-sheets, and is crucial to the function of Cas9 by recognizing a specific motif termed the repeat:anti-repeat region of the single guide RNA and DNA complex. The REC-2 domain contains six α-helix’s in a bundle but there is no current understanding of its function. | ||
Revision as of 20:46, 13 October 2015
|

FunctionFunction
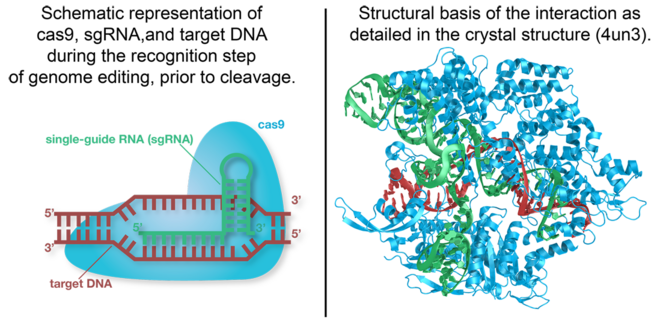
Cas9 is the RNA-guided DNA endonuclease used by the CRISPR (clustered regularly interspaced short palindromic repeats)-associated systems to generate double-strand DNA breaks in the invading DNA during an adaptive bacterial immune response. Three different types of CRISPR mechanisms have been discovered, however, only type II CRISPR systems are heavily researched. In vivo, Cas9 requires CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) to guide the endonuclease toward invading DNA based on the complementary sequence recognition of these RNAs. Cas9 then targets and cleaves foreign DNA to interfere with viral replication. The CRISPR-associated endonuclease has been exploited for use in genome editing systems. In such systems, an engineered single-guide RNA (sgRNA) is used to perform the function of crRNA-tracRNA complex to target double-stranded breaks in genomic DNA. Depending on what repair pathway is triggered, often dictated by the inclusion of additional engineered components, the targeted site either is disrupted or incorporates additional genetic sequences. [1]
Structural highlightsStructural highlights
Cas9 is a structurally bilobed, containing specific domains for the recognition of target DNA and nucleases to cleave DNA strands. Each of these lobes contain three major units essential for a functional endonuclease, these are the essential .
The recognition lobe (REC) contains a long bridge helix, the REC-1 domain and the REC-2 domain. It is also the least conserved lobe through the types of Cas9. The long α-helix bridge, which is arginine-rich, is essential for recognizing single guide RNA-DNA complexes on target DNA. This structure has been shown to be conserved through Cas9 proteins. The REC-1 domain contains 25 α-helixes and two β-sheets, and is crucial to the function of Cas9 by recognizing a specific motif termed the repeat:anti-repeat region of the single guide RNA and DNA complex. The REC-2 domain contains six α-helix’s in a bundle but there is no current understanding of its function.
The nuclease lobe (NUC) contains a RuvC domain, HNH domain and the PAM-interacting domain (PI). The RuvC domain is comprised of three RuvC motifs that are made up of two-stranded antiparallel β-sheets, and six-stranded β-sheets, which are flanked by nine α-helices. The RuvC nuclease cleaves the non-complementary, single stranded DNA. The HNH domain is composed of a two-stranded antiparallel β-sheet which is flanked by four α-helixes and cleaves the complementary strand of target DNA. The PI is made up of seven α-helixes and numerous strand-varying antiparallel β-sheets which recognize the PAM sequence on the non-complementary target DNA strand.[2]
DNA InteractionDNA Interaction
Target DNA contains a protospacer adjacent motif on the non-complementary strand, which constitues the . This canonical sequence of 5’-NGG-3’ is recognized by Cas9 and is essential for genetic interference and editing, therefore, it will not cleave the target sequence if the PAM sequence is absent. The double Guanine of the non-complementary sequence strand interacts via hydrogen bonding from the major groove through two conserved arginine residues on the carboxy-terminus of Cas9 [3]. The minor groove of the PAM sequence interacts with a serine, through a hydrogen bridge to the last guanine, and lysine residue on the complementary target strand of the middle guanine.
The deoxyribose-phosphate backbone of the non-complementary strand is arranged in close proximity to various hydrogen bonding atoms, some of which are accomplished through water molecules, and ionic interactions. A phosphate lock loop provides local strand separation upstream of the PAM sequence when a Lysine and Serine residue stabilize target DNA. The PAM sequence recognition of Cas9 is an integral function of its specific binding, subsequent base pair melting and cleavage of target DNA.
3D structures of Cas93D structures of Cas9
Streptococcus pyogenes Cas9Streptococcus pyogenes Cas9
- 4un3, 4un4, and 4un5 - S. pyogenes Cas9 bound to sgRNA and target DNA
- 4oo8 - S. pyogenes Cas9 bound to sgRNA and target DNA
- 4cmp
- 4cmq - Mn2+-bound S. pyogenes Cas9
Actinomyces naeslundii Cas9Actinomyces naeslundii Cas9
See AlsoSee Also
ReferencesReferences
- ↑ Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014 Jun 5;157(6):1262-78. doi: 10.1016/j.cell.2014.05.010. PMID:24906146 doi:http://dx.doi.org/10.1016/j.cell.2014.05.010
- ↑ Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, Dohmae N, Ishitani R, Zhang F, Nureki O. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 2014 Feb 27;156(5):935-49. doi: 10.1016/j.cell.2014.02.001. Epub 2014 Feb, 13. PMID:24529477 doi:http://dx.doi.org/10.1016/j.cell.2014.02.001
- ↑ Anders C, Niewoehner O, Duerst A, Jinek M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 2014 Jul 27. doi: 10.1038/nature13579. PMID:25079318 doi:http://dx.doi.org/10.1038/nature13579