2abx: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
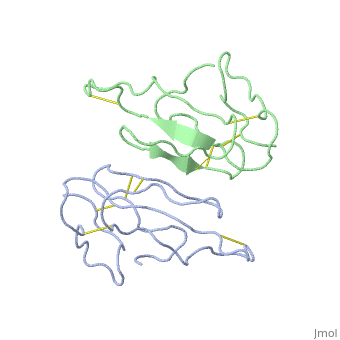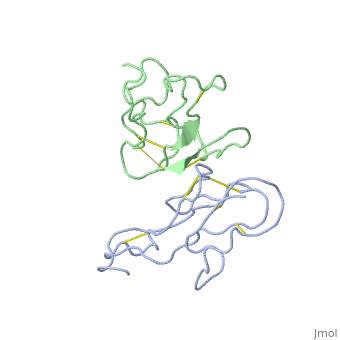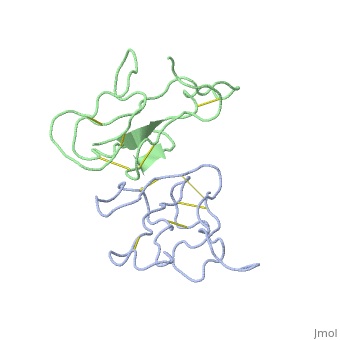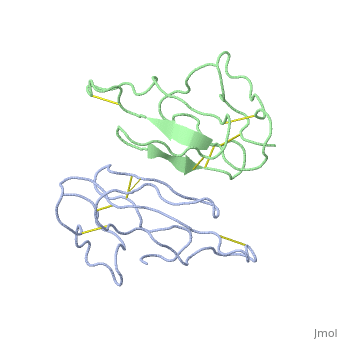
<StructureSection load='2abx' size='340' side='right' caption='[[2abx]], [[Resolution|resolution]] 2.50Å' scene=''> | <StructureSection load='2abx' size='340' side='right' caption='[[2abx]], [[Resolution|resolution]] 2.50Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
<table><tr><td colspan='2'>[[2abx]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/ | <table><tr><td colspan='2'>[[2abx]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Bunmu Bunmu]. This structure supersedes the now removed PDB entry [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=1abx 1abx]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2ABX OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2ABX FirstGlance]. <br> | ||
</td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2abx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2abx OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2abx RCSB], [http://www.ebi.ac.uk/pdbsum/2abx PDBsum]</span></td></tr> | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2abx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2abx OCA], [http://pdbe.org/2abx PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=2abx RCSB], [http://www.ebi.ac.uk/pdbsum/2abx PDBsum]</span></td></tr> | ||
</table> | </table> | ||
== Function == | == Function == | ||
| Line 25: | Line 25: | ||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
</div> | </div> | ||
<div class="pdbe-citations 2abx" style="background-color:#fffaf0;"></div> | |||
==See Also== | ==See Also== | ||
| Line 32: | Line 33: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
[[Category: | [[Category: Bunmu]] | ||
[[Category: Love, R]] | [[Category: Love, R]] | ||
[[Category: Stroud, R]] | [[Category: Stroud, R]] | ||
[[Category: Postsynaptic neurotoxin]] | [[Category: Postsynaptic neurotoxin]] | ||
Revision as of 04:46, 11 September 2015
THE CRYSTAL STRUCTURE OF ALPHA-BUNGAROTOXIN AT 2.5 ANGSTROMS RESOLUTION. RELATION TO SOLUTION STRUCTURE AND BINDING TO ACETYLCHOLINE RECEPTORTHE CRYSTAL STRUCTURE OF ALPHA-BUNGAROTOXIN AT 2.5 ANGSTROMS RESOLUTION. RELATION TO SOLUTION STRUCTURE AND BINDING TO ACETYLCHOLINE RECEPTOR
Structural highlights
Function[NXL1A_BUNMU] Binds with high affinity to muscular and neuronal (alpha-7, alpha-8, and alpha-9) nicotinic acetylcholine receptors. Produces peripheral paralysis by blocking neuromuscular transmission at the postsynaptic site. Blocks the extracellular increase of dopamine evoked by nicotine only at the higher dose (4.2 uM).[1] [2] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedWe report collection of 2.5 A resolution X-ray diffraction data from newly grown crystals of the rare 'small unit cell' form of the long snake neurotoxin, alpha-bungarotoxin. The previous model of the molecule has been rebuilt, and refined using least-square methods to a crystallographic residual of 0.24 at 2.5 A resolution. alpha-Bungarotoxin's crystal structure is compared with the crystal structures of two other snake neurotoxins (cobratoxin and erabutoxin), and with its solution structure inferred from spectroscopic studies. Significant differences include less beta-sheet in bungarotoxin's crystal structure than in solution, or in the crystal structures of other neurotoxins, and an unusual orientation in the crystal of the invariant tryptophan. The functional, binding surface of bungarotoxin is described; it consists primarily of hydrophobic and hydrogen-bonding groups and only a few charged side-chains. The structure is compared with experimental binding parameters for neurotoxins. The crystal structure of alpha-bungarotoxin at 2.5 A resolution: relation to solution structure and binding to acetylcholine receptor.,Love RA, Stroud RM Protein Eng. 1986 Oct-Nov;1(1):37-46. PMID:3507686[3] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||