PLC beta 3 Gq: Difference between revisions
Shir Navot (talk | contribs) New page: ==Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q== <StructureSection load='3ohm' size='340' side='right' caption='Caption for this structure' scene=''> This is ... |
Shir Navot (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
==Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q== | ==Unique bidirectional interactions of Phospholipase C beta 3 with G alpha Q== | ||
<StructureSection load='3ohm' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='3ohm' size='340' side='right' caption='Caption for this structure' scene=''> | ||
== Introduction == | |||
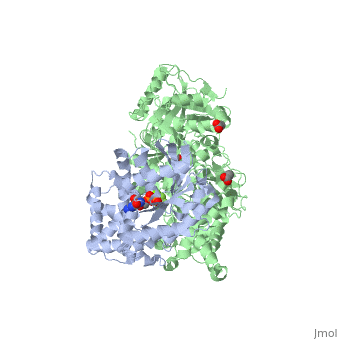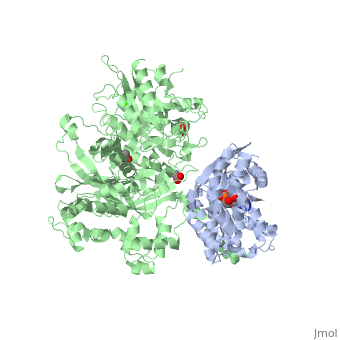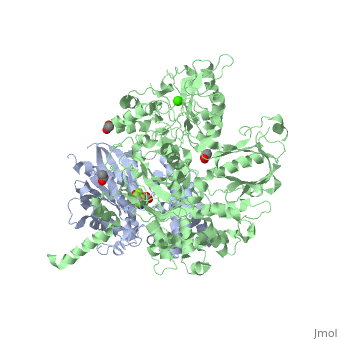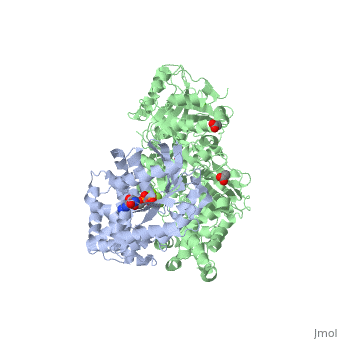
Phospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate [IP2] to the second messengers inositol 1,4,5-trisphosphate [IP3] and diacylglycerol [DAG] in an essential step for the physiological action of many hormones, neurotransmitters, growth factors, and other extracellular stimuli. These cascades use signaling complexes consisting of G alpha subunits of the Gq family of heterotrimeric guanine nucleotide–binding proteins (G proteins) and PLC-beta isozymes (β1-4). Agonist-stimulated receptors increase exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Gαq. GTP-bound Gαq engages and activates PLC- β3, and PLC- β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism when the PLC-β3 enzyme has the ability to terminate the Gαq protein signal in addition to being activated by it.<ref>PMID:20966218</ref> <ref>PMID:23880553</ref> | |||
== Structural highlights == | == Structural highlights == | ||
Revision as of 14:39, 5 May 2015
Unique bidirectional interactions of Phospholipase C beta 3 with G alpha QUnique bidirectional interactions of Phospholipase C beta 3 with G alpha Q
IntroductionPhospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate [IP2] to the second messengers inositol 1,4,5-trisphosphate [IP3] and diacylglycerol [DAG] in an essential step for the physiological action of many hormones, neurotransmitters, growth factors, and other extracellular stimuli. These cascades use signaling complexes consisting of G alpha subunits of the Gq family of heterotrimeric guanine nucleotide–binding proteins (G proteins) and PLC-beta isozymes (β1-4). Agonist-stimulated receptors increase exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) on Gαq. GTP-bound Gαq engages and activates PLC- β3, and PLC- β3 increases up to three orders of magnitude the rate of hydrolysis of GTP by its activating G protein. This is a unique mechanism when the PLC-β3 enzyme has the ability to terminate the Gαq protein signal in addition to being activated by it.[1] [2] Structural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ Waldo GL, Ricks TK, Hicks SN, Cheever ML, Kawano T, Tsuboi K, Wang X, Montell C, Kozasa T, Sondek J, Harden TK. Kinetic Scaffolding Mediated by a Phospholipase C-{beta} and Gq Signaling Complex. Science. 2010 Nov 12;330(6006):974-80. Epub 2010 Oct 21. PMID:20966218 doi:10.1126/science.1193438
- ↑ Lyon AM, Tesmer JJ. Structural insights into phospholipase C-beta function. Mol Pharmacol. 2013 Oct;84(4):488-500. doi: 10.1124/mol.113.087403. Epub 2013 Jul, 23. PMID:23880553 doi:http://dx.doi.org/10.1124/mol.113.087403