Recombinase A: Difference between revisions
Taylor Light (talk | contribs) No edit summary |
Taylor Light (talk | contribs) No edit summary |
||
| Line 12: | Line 12: | ||
== Binding Sites on RecA == | == Binding Sites on RecA == | ||
As RecA has many different functions, it also has several different binding sites for DNA, ATP, the LexA repressor, the λ repressor, as well as other RecA protein monomers to form a variety of oligomers.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> <ref name=Walker> Walker, J. E.; Saraste, M.; Runswick, M. J. Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945-951. PMCID: PMC553140 </ref> There are six RecA proteins per turn of the helix, and each individual monomer is capable of binding three base pairs of the extended conformation of DNA.<ref name=Cox> Cox, M. M. Motoring along with the bacterial RecA protein. Nat. Rev. Mol. Cell Biol. 2007, 8, 127-138. DOI: 10.1038/nrm2099 </ref> This filament is not the only oligomer of RecA that exists in solution, however. Sattin and Goh have reported a variety of RecA structures in buffer, such as monomers, hexamers, rods/fibrils, protofibrils, and other small aggregates.<ref name=Sattin> Sattin, B. D.; Goh, M. C. Novel polymorphism of recA fibrils revealed by Atomic Force Microscopy. J. Biol. Phys. 2006, 32, 153-168. DOI: 10.1007/s10867-006-9010-3 </ref> Moreover, the type and amount of these different aggregation states is dynamic. Brenner and Zlotnick reported that the presence of monovalent salts changed the distribution of RecA aggregation states and that higher protein concentration tended to correspond to more aggregated structures.<ref name=Brenner> Brenner, S. L.; Zlotnick, A. RecA Protein Self-assembly: Multiple Discrete Aggregation States. J. Mol. Biol. 1988, 204, 959-972. DOI: 10.1016/0022-2836(88)90055-1 </ref> ATP hydrolysis occurs in the region of a loop consisting of amino acids 66-73 of the protein. This loop, which corresponds to the Walker A box motif, has the sequence GPESSGKT.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> This sequence corresponds to a variation known as the phosphate binding loop, which has a sequence [G/A]XXXXGK[T/S] found in many nucleoside triphosphate (NTP)-binding proteins.<ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> <ref name=Walker> Walker, J. E.; Saraste, M.; Runswick, M. J. Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945-951. PMCID: PMC553140 </ref> Several of the residues in this phosphate binding loop can be seen interacting with the β and γ phosphates of ATP in the ATP-binding site proposed by Story and Steitz.<ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> The binding of various ligands to RecA has been shown to change the pitch, the “distance covered by each turn of the helix,” of the protein filament.<ref name=Ellouze> Ellouze, C.; Takahashi, M.; Wittung, P.; Mortensen, K.; Schnarr, M.; Nordén, B. Evidence for elongation of helical pitch of the helical pitch of the RecA filament upon ATP and ADP binding using small-angle neutron scattering. Eur. J. Biochem. 1995,233, 579-583. DOI: 10.1111/j.1432-1033.1995.579_2.x </ref> <ref name=Menetski> Menetski, J. P.; Kowalczykowski, S. C. Interaction of recA protein with single-stranded DNA: Quantitative aspects of binding affinity modulation by nucleotide cofactors. J. Mol. Biol. 1985, 181, 281-295. DOI: 10.1016/0022-2836(85)90092-0 </ref> RecA in the absence of any cofactor is in a “closed” conformation with a helical pitch of 7 nm (DNA binding to the RecA does not alter the pitch significantly). RecA bound to ATP increases the pitch to 9 nm.<ref name= | As RecA has many different functions, it also has several different binding sites for DNA, ATP, the LexA repressor, the λ repressor, as well as other RecA protein monomers to form a variety of oligomers.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> <ref name=Walker> Walker, J. E.; Saraste, M.; Runswick, M. J. Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945-951. PMCID: PMC553140 </ref> There are six RecA proteins per turn of the helix, and each individual monomer is capable of binding three base pairs of the extended conformation of DNA.<ref name=Cox> Cox, M. M. Motoring along with the bacterial RecA protein. Nat. Rev. Mol. Cell Biol. 2007, 8, 127-138. DOI: 10.1038/nrm2099 </ref> This filament is not the only oligomer of RecA that exists in solution, however. Sattin and Goh have reported a variety of RecA structures in buffer, such as monomers, hexamers, rods/fibrils, protofibrils, and other small aggregates.<ref name=Sattin> Sattin, B. D.; Goh, M. C. Novel polymorphism of recA fibrils revealed by Atomic Force Microscopy. J. Biol. Phys. 2006, 32, 153-168. DOI: 10.1007/s10867-006-9010-3 </ref> Moreover, the type and amount of these different aggregation states is dynamic. Brenner and Zlotnick reported that the presence of monovalent salts changed the distribution of RecA aggregation states and that higher protein concentration tended to correspond to more aggregated structures.<ref name=Brenner> Brenner, S. L.; Zlotnick, A. RecA Protein Self-assembly: Multiple Discrete Aggregation States. J. Mol. Biol. 1988, 204, 959-972. DOI: 10.1016/0022-2836(88)90055-1 </ref> ATP hydrolysis occurs in the region of a loop consisting of amino acids 66-73 of the protein. This loop, which corresponds to the Walker A box motif, has the sequence GPESSGKT.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> This sequence corresponds to a variation known as the phosphate binding loop, which has a sequence [G/A]XXXXGK[T/S] found in many nucleoside triphosphate (NTP)-binding proteins.<ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> <ref name=Walker> Walker, J. E.; Saraste, M.; Runswick, M. J. Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945-951. PMCID: PMC553140 </ref> Several of the residues in this phosphate binding loop can be seen interacting with the β and γ phosphates of ATP in the ATP-binding site proposed by Story and Steitz.<ref name=Story> Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0 </ref> The binding of various ligands to RecA has been shown to change the pitch, the “distance covered by each turn of the helix,” of the protein filament.<ref name=Ellouze> Ellouze, C.; Takahashi, M.; Wittung, P.; Mortensen, K.; Schnarr, M.; Nordén, B. Evidence for elongation of helical pitch of the helical pitch of the RecA filament upon ATP and ADP binding using small-angle neutron scattering. Eur. J. Biochem. 1995,233, 579-583. DOI: 10.1111/j.1432-1033.1995.579_2.x </ref> <ref name=Menetski> Menetski, J. P.; Kowalczykowski, S. C. Interaction of recA protein with single-stranded DNA: Quantitative aspects of binding affinity modulation by nucleotide cofactors. J. Mol. Biol. 1985, 181, 281-295. DOI: 10.1016/0022-2836(85)90092-0 </ref> RecA in the absence of any cofactor is in a “closed” conformation with a helical pitch of 7 nm (DNA binding to the RecA does not alter the pitch significantly). RecA bound to ATP increases the pitch to 9 nm.<ref name=Ellouze> Ellouze, C.; Takahashi, M.; Wittung, P.; Mortensen, K.; Schnarr, M.; Nordén, B. Evidence for elongation of helical pitch of the helical pitch of the RecA filament upon ATP and ADP binding using small-angle neutron scattering. Eur. J. Biochem. 1995,233, 579-583. DOI: 10.1111/j.1432-1033.1995.579_2.x </ref> This RecA-ATP structure is marked by a higher affinity for DNA.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref>'''k15''' However, the binding of ADP to RecA only raises the pitch to 8.2 nm,<ref name=Ellouze> Ellouze, C.; Takahashi, M.; Wittung, P.; Mortensen, K.; Schnarr, M.; Nordén, B. Evidence for elongation of helical pitch of the helical pitch of the RecA filament upon ATP and ADP binding using small-angle neutron scattering. Eur. J. Biochem. 1995,233, 579-583. DOI: 10.1111/j.1432-1033.1995.579_2.x </ref> the conformation of which is known to have a lower affinity for DNA.<ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref> <ref name=Roca> Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3 </ref>'''k15''' | ||
== RecA and Hofmeister Salts == | == RecA and Hofmeister Salts == | ||
Revision as of 07:18, 26 February 2015
|
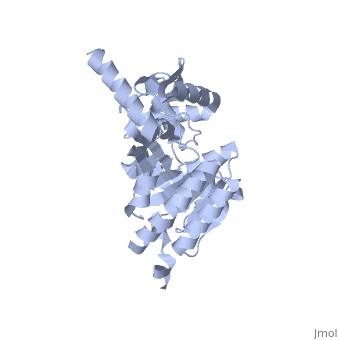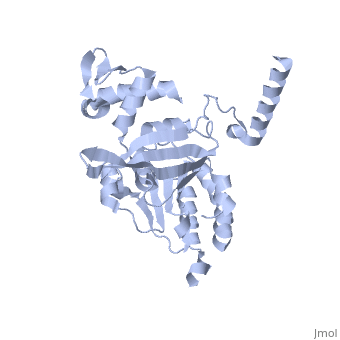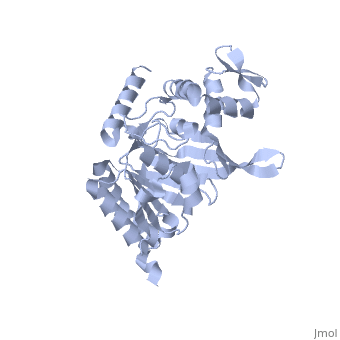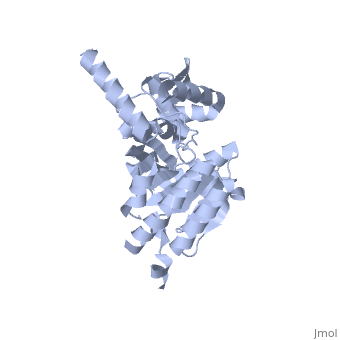
Recombinase A (RecA), a naturally aggregating 352 amino acid protein involved in DNA repair, is an important asset to the genetic integrity of the Escherichia coli (E. coli) genome.[1] The survival of all species rely on such DNA repair processes. RecA homologues are found in all kingdoms including archaebacteria, eubacteria, and eukaryotes.[2] RAD51, for example, is a RecA homologue found specifically in humans.[3] An over-expression of RAD51 in the nuclei of tumor cells when compared to those of normal breast tissue has been linked to sporadic, non-hereditary, breast cancers.[4]
DNA RepairDNA Repair
In E. coli, RecA’s central function involves strand exchange, specifically recombinational DNA repair.[5] The DNA recombination mechanism for RecA is a process that results in the exchange of strands between two homologous DNA molecules.[6] The DNA that results from this process is a nicked circular dsDNA molecule and one or two linear ssDNA molecules, depending on the number of DNA strands involved (three or four).[5] [6] During DNA strand exchange, adenosine triphosphate (ATP) is hydrolyzed to form adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP hydrolysis is required for DNA strand exchange to be unidirectional (without ATP hydrolysis, strand exchange is also bidirectional), for the circumvention of various structural obstacles on the DNA molecule such as heterologous inserts, and for DNA strand exchange to occur with four stands of DNA.[1] [5]
Other RecA FunctionsOther RecA Functions
RecA is also involved in inducing the SOS response to DNA damage by assisting in the cleavage, and consequent inactivation, of proteins.[5] Examples of such proteins are the LexA repressor and the λ repressor.[5]
Binding Sites on RecABinding Sites on RecA
As RecA has many different functions, it also has several different binding sites for DNA, ATP, the LexA repressor, the λ repressor, as well as other RecA protein monomers to form a variety of oligomers.[5] [7] [8] There are six RecA proteins per turn of the helix, and each individual monomer is capable of binding three base pairs of the extended conformation of DNA.[9] This filament is not the only oligomer of RecA that exists in solution, however. Sattin and Goh have reported a variety of RecA structures in buffer, such as monomers, hexamers, rods/fibrils, protofibrils, and other small aggregates.[10] Moreover, the type and amount of these different aggregation states is dynamic. Brenner and Zlotnick reported that the presence of monovalent salts changed the distribution of RecA aggregation states and that higher protein concentration tended to correspond to more aggregated structures.[11] ATP hydrolysis occurs in the region of a loop consisting of amino acids 66-73 of the protein. This loop, which corresponds to the Walker A box motif, has the sequence GPESSGKT.[5] [7] This sequence corresponds to a variation known as the phosphate binding loop, which has a sequence [G/A]XXXXGK[T/S] found in many nucleoside triphosphate (NTP)-binding proteins.[7] [8] Several of the residues in this phosphate binding loop can be seen interacting with the β and γ phosphates of ATP in the ATP-binding site proposed by Story and Steitz.[7] The binding of various ligands to RecA has been shown to change the pitch, the “distance covered by each turn of the helix,” of the protein filament.[12] [13] RecA in the absence of any cofactor is in a “closed” conformation with a helical pitch of 7 nm (DNA binding to the RecA does not alter the pitch significantly). RecA bound to ATP increases the pitch to 9 nm.[12] This RecA-ATP structure is marked by a higher affinity for DNA.[5] [5]k15 However, the binding of ADP to RecA only raises the pitch to 8.2 nm,[12] the conformation of which is known to have a lower affinity for DNA.[5] [5]k15
RecA and Hofmeister SaltsRecA and Hofmeister Salts
High salt concentrations have also been shown to be able to elongate the RecA protein filament as well. Petukhov et al. demonstrated that a high concentration of NaCl increased the helical pitch from 7.8 to 8.6 nm.[5] k16 Thus, high salt concentrations appear to induce the active (stretched) form of RecA in the absence of DNA.[5] k16 Other studies have found that the free Magnesium ion binds to RecA and extends the filament more than 150% compared to the filament when DNA is bound.[5] {Lusetti} Moreover, although normally RecA requires DNA to hydrolyze ATP, high salt concentrations are able to stimulate ATP hydrolysis in the absence of DNA.[5] k17 Brenner and Zlotnick reported that the presence of monovalent salts changed the distribution of RecA aggregation states and that the more aggregated structures corresponded to higher protein concentration.[11] Previous studies have shown that various Hofmeister salts affect the secondary structure, stability, and aggregation behavior of RecA differently.[11] [5] T12 Additionally, RecA has been demonstrated to follow the inverse-anionic Hofmeister series and the presence of some ions promotes nonspecific aggregation.[5] T12
ReferencesReferences
- ↑ 1.0 1.1 Shan, Q.; Cox, M. M.; Inman, R. B. DNA Strand Exchange Promoted by RecA K72R. J. Biol. Chem. 1996, 271, 5712-5724. DOI:10.1074/jbc.271.10.5712
- ↑ Brendel, V.; Brocchieri, L.; Sandler, S.J.; Clark, A.J.; Karlin, S. Evolutionary comparisons of RecA-like proteins across all major kingdoms of living organisms. J. Mol. Evol. 1997, 44, 528-541. DOI: 10.1007/PL00006177
- ↑ Baumann, P.; Benson, F. E.; West, S. C. Human Rad51 Protein Promotes ATP-Dependent Homologous Pairing and Strand Transfer Reactions in Vitro. Cell. 1996, 87, 757-766. DOI: 10.1016/S0092-8674(00)81394-X
- ↑ Maacke, H.; Opitz, S.; Jost, K.; Hamdorf, W.; Henning, W. Krüger, S. Feller, A.C.; Lopens, A.; Diedrich, K.; Schwinger, E.; Stürzbecher, H.W. Over-expression of wild-type Rad51 correlates with histological grading of invasive ductal breast cancer. Int. J. Cancer. 2000, 88, 907-913. DOI: 10.1002/1097-0215(20001215)88:63.0.CO;2-4
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 Roca, A. I.; Cox, M. M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129-223. DOI: 10.1016/S0079-6603(08)61005-3
- ↑ 6.0 6.1 Nayak, S.; Hildebrand, E.L.; Bryant, F.R. ADP-dependent DNA strand exchange by the Mutant RecA protein. J. Biol. Chem.2001, 276, 14933-14938. DOI:10.1074/jbc.M100470200
- ↑ 7.0 7.1 7.2 7.3 Story, R. M.; Weber, I. T.; Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature (London) 1992, 355, 318-325. DOI: 10.1038/355318a0
- ↑ 8.0 8.1 Walker, J. E.; Saraste, M.; Runswick, M. J. Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982, 1, 945-951. PMCID: PMC553140
- ↑ Cox, M. M. Motoring along with the bacterial RecA protein. Nat. Rev. Mol. Cell Biol. 2007, 8, 127-138. DOI: 10.1038/nrm2099
- ↑ Sattin, B. D.; Goh, M. C. Novel polymorphism of recA fibrils revealed by Atomic Force Microscopy. J. Biol. Phys. 2006, 32, 153-168. DOI: 10.1007/s10867-006-9010-3
- ↑ 11.0 11.1 11.2 Brenner, S. L.; Zlotnick, A. RecA Protein Self-assembly: Multiple Discrete Aggregation States. J. Mol. Biol. 1988, 204, 959-972. DOI: 10.1016/0022-2836(88)90055-1
- ↑ 12.0 12.1 12.2 Ellouze, C.; Takahashi, M.; Wittung, P.; Mortensen, K.; Schnarr, M.; Nordén, B. Evidence for elongation of helical pitch of the helical pitch of the RecA filament upon ATP and ADP binding using small-angle neutron scattering. Eur. J. Biochem. 1995,233, 579-583. DOI: 10.1111/j.1432-1033.1995.579_2.x
- ↑ Menetski, J. P.; Kowalczykowski, S. C. Interaction of recA protein with single-stranded DNA: Quantitative aspects of binding affinity modulation by nucleotide cofactors. J. Mol. Biol. 1985, 181, 281-295. DOI: 10.1016/0022-2836(85)90092-0
--Taylor Light 22:30, 25 February 2015 (IST)
3D Structures of Recombinase A3D Structures of Recombinase A
Updated on 26-February-2015