1uyn: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
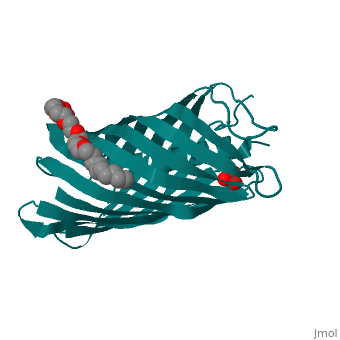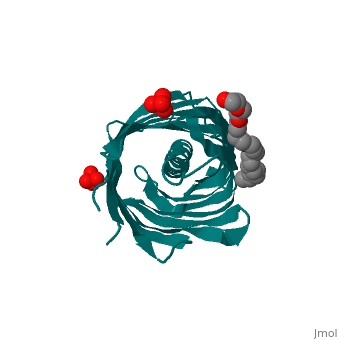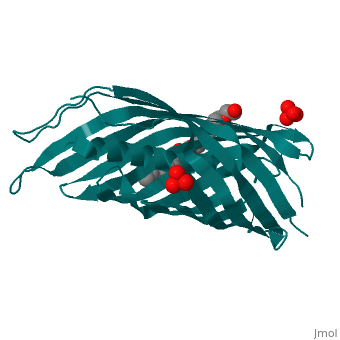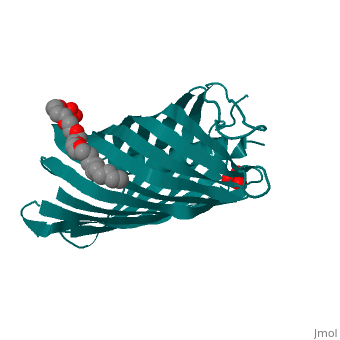
Autotransporters are virulence-related proteins of Gram-negative bacteria, that are secreted via an outer-membrane-based C-terminal extension, the, translocator domain. This domain supposedly is sufficient for the, transport of the N-terminal passenger domain across the outer membrane. We, present here the crystal structure of the in vitro-folded translocator, domain of the autotransporter NalP from Neisseria meningitidis, which, reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A, that is filled by an N-terminal alpha-helix. The domain has pore activity, in vivo and in vitro. Our data are consistent with the model of, passenger-domain transport through the hydrophilic channel within the, beta-barrel, and inconsistent with a model for transport through a central, ... | Autotransporters are virulence-related proteins of Gram-negative bacteria, that are secreted via an outer-membrane-based C-terminal extension, the, translocator domain. This domain supposedly is sufficient for the, transport of the N-terminal passenger domain across the outer membrane. We, present here the crystal structure of the in vitro-folded translocator, domain of the autotransporter NalP from Neisseria meningitidis, which, reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A, that is filled by an N-terminal alpha-helix. The domain has pore activity, in vivo and in vitro. Our data are consistent with the model of, passenger-domain transport through the hydrophilic channel within the, beta-barrel, and inconsistent with a model for transport through a central, channel formed by an oligomer of translocator domains. However, the, dimensions of the pore imply translocation of the secreted domain in an, unfolded form. An alternative model, possibly covering the transport of, folded domains, is that passenger-domain transport involves the Omp85, complex, the machinery required for membrane insertion of outer-membrane, proteins, on which autotransporters are dependent. | ||
==About this Structure== | ==About this Structure== | ||
1UYN is a | 1UYN is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Neisseria_meningitidis Neisseria meningitidis] with SO4 and CXE as [http://en.wikipedia.org/wiki/ligands ligands]. Structure known Active Site: AC1. Full crystallographic information is available from [http://ispc.weizmann.ac.il/oca-bin/ocashort?id=1UYN OCA]. | ||
==Reference== | ==Reference== | ||
| Line 29: | Line 29: | ||
[[Category: translocator domain]] | [[Category: translocator domain]] | ||
''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on Mon Nov 5 13:43:09 2007'' | ||
Revision as of 14:37, 5 November 2007
|
TRANSLOCATOR DOMAIN OF AUTOTRANSPORTER NALP FROM NEISSERIA MENINGITIDIS
OverviewOverview
Autotransporters are virulence-related proteins of Gram-negative bacteria, that are secreted via an outer-membrane-based C-terminal extension, the, translocator domain. This domain supposedly is sufficient for the, transport of the N-terminal passenger domain across the outer membrane. We, present here the crystal structure of the in vitro-folded translocator, domain of the autotransporter NalP from Neisseria meningitidis, which, reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A, that is filled by an N-terminal alpha-helix. The domain has pore activity, in vivo and in vitro. Our data are consistent with the model of, passenger-domain transport through the hydrophilic channel within the, beta-barrel, and inconsistent with a model for transport through a central, channel formed by an oligomer of translocator domains. However, the, dimensions of the pore imply translocation of the secreted domain in an, unfolded form. An alternative model, possibly covering the transport of, folded domains, is that passenger-domain transport involves the Omp85, complex, the machinery required for membrane insertion of outer-membrane, proteins, on which autotransporters are dependent.
About this StructureAbout this Structure
1UYN is a Single protein structure of sequence from Neisseria meningitidis with SO4 and CXE as ligands. Structure known Active Site: AC1. Full crystallographic information is available from OCA.
ReferenceReference
Structure of the translocator domain of a bacterial autotransporter., Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P, EMBO J. 2004 Mar 24;23(6):1257-66. Epub 2004 Mar 11. PMID:15014442
Page seeded by OCA on Mon Nov 5 13:43:09 2007