4eb6: Difference between revisions
No edit summary |
No edit summary |
||
| Line 8: | Line 8: | ||
<tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4eb6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4eb6 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=4eb6 RCSB], [http://www.ebi.ac.uk/pdbsum/4eb6 PDBsum]</span></td></tr> | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4eb6 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4eb6 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=4eb6 RCSB], [http://www.ebi.ac.uk/pdbsum/4eb6 PDBsum]</span></td></tr> | ||
</table> | </table> | ||
== Function == | |||
[[http://www.uniprot.org/uniprot/D0VWZ0_SHEEP D0VWZ0_SHEEP]] Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505][SAAS:SAAS023123_004_019801] [[http://www.uniprot.org/uniprot/STMN4_RAT STMN4_RAT]] Exhibits microtubule-destabilizing activity.<ref>PMID:15039434</ref> <ref>PMID:12111843</ref> <ref>PMID:15014504</ref> [[http://www.uniprot.org/uniprot/D0VWY9_SHEEP D0VWY9_SHEEP]] Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505] | |||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
Revision as of 16:53, 25 December 2014
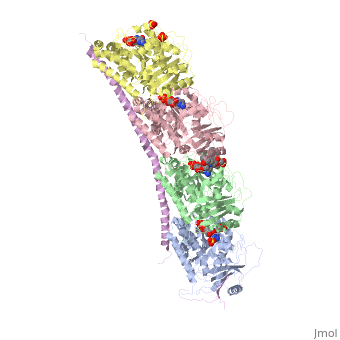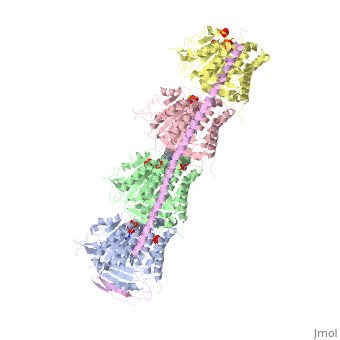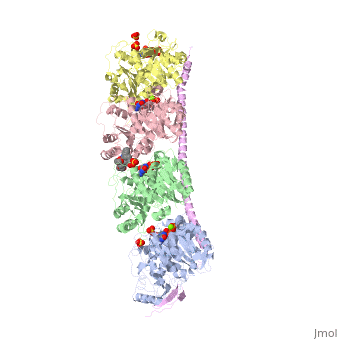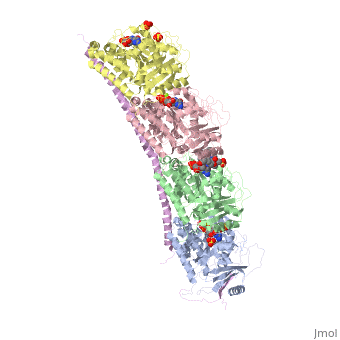
Tubulin-Vinblastine: Stathmin-like complexTubulin-Vinblastine: Stathmin-like complex
Structural highlights
Function[D0VWZ0_SHEEP] Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505][SAAS:SAAS023123_004_019801] [STMN4_RAT] Exhibits microtubule-destabilizing activity.[1] [2] [3] [D0VWY9_SHEEP] Tubulin is the major constituent of microtubules. It binds two moles of GTP, one at an exchangeable site on the beta chain and one at a non-exchangeable site on the alpha chain (By similarity).[RuleBase:RU003505] Publication Abstract from PubMedVinca-domain ligands are compounds that bind to tubulin at its inter-heterodimeric interface and favour heterogeneous protofilament-like assemblies, giving rise to helices and rings. This is the basis for their inhibition of microtubule assembly, for their antimitotic activities and for their use in anticancer chemotherapy. Ustiloxins are vinca-domain ligands with a well established total synthesis. A 2.7 A resolution structure of ustiloxin D bound to the vinca domain embedded in the complex of two tubulins with the stathmin-like domain of RB3 (T(2)R) has been determined. This finding precisely defines the interactions of ustiloxins with tubulin and, taken together with structures of other vinca-ligand complexes, allows structure-based suggestions to be made for improved activity. These comparisons also provide a rationale for the large-scale polymorphism of the protofilament-like assemblies mediated by vinca-domain ligands based on local differences in their interactions with the two tubulin heterodimers constituting their binding site. Structural plasticity of tubulin assembly probed by vinca-domain ligands.,Ranaivoson FM, Gigant B, Berritt S, Joullie M, Knossow M Acta Crystallogr D Biol Crystallogr. 2012 Aug;68(Pt 8):927-34. Epub 2012 Jul 7. PMID:22868758[4] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||||