Pheromone binding protein: Difference between revisions
Nurit Eliash (talk | contribs) No edit summary |
Nurit Eliash (talk | contribs) No edit summary |
||
| Line 16: | Line 16: | ||
<scene name='60/609542/Glycerol/2'>Glycerol</scene> | <scene name='60/609542/Glycerol/2'>Glycerol</scene> | ||
<scene name='60/609542/Binding_site/ | <scene name='60/609542/Binding_site/3'>hydrophobic residues</scene> | ||
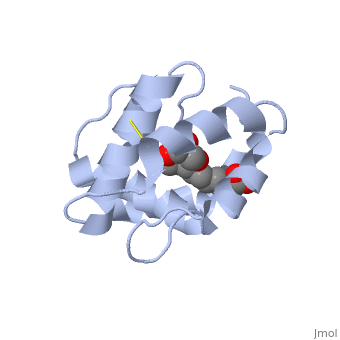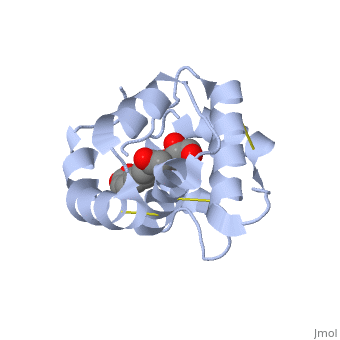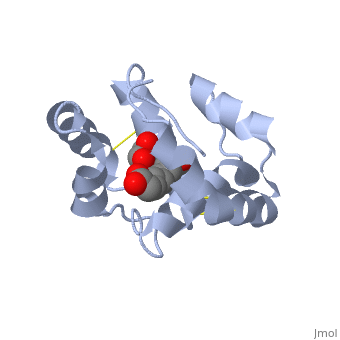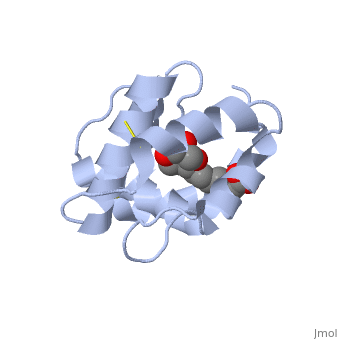
Pheromone-binding protein [http://www.uniprot.org/uniprot/Q9U9J6/ ASP] of the honeybee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] L. (Hymenoptera: Apidea) was first isolated and characterized by Danty ''et al''. (1998)<ref>DOI:10.1016/j.jmb.2008.04.048</ref> from the bee antennae. | Pheromone-binding protein [http://www.uniprot.org/uniprot/Q9U9J6/ ASP] of the honeybee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] L. (Hymenoptera: Apidea) was first isolated and characterized by Danty ''et al''. (1998)<ref>DOI:10.1016/j.jmb.2008.04.048</ref> from the bee antennae. | ||
Revision as of 14:12, 22 November 2014
IntroductionIntroduction
As the most ancient sense in nature, smell and chemical communication play a major role in successful mating, host and selection and other essential behaviors. The detection of volatiles (often a small hydrophobic molecules) starts by Pheromone binding proteins are soluble proteins invovled in the early stages of pheromone detection. As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee. Chemical communication is crucial in social insects, where a complicated and delicate system of signals must be maintained in order to preserve the fragile equilibrium between the queen and the workers. In the hive of the honey bee Apis mellifera this equilibrium exists partially due to the extraction of blend of substances called queen mandibular pheromone (QMP), by the queen. The three major component of the QMP blend are: 9-keto-2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decenoic acid (9-HDA R-(−) or S-(+)).
Pheromone-binding protein ASP of the honeybee Apis mellifera L. (Hymenoptera: Apidea) was first isolated and characterized by Danty et al. (1998)[1] from the bee antennae. or to the article describing Jmol [2] to the rescue. StructureThe protein has a 1 alpha chain, that can be seen in pink.
FunctionThe protein ligand, 9-ODA File:9-ODA.jpg Structural highlightsThis is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
| ||||||||||
ReferencesReferences
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Tegoni M, Cambillau C. Structural basis of the honey bee PBP pheromone and pH-induced conformational change. J Mol Biol. 2008 Jun 27;380(1):158-69. Epub 2008 Apr 27. PMID:18508083 doi:10.1016/j.jmb.2008.04.048
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644