Molecular Playground/Streptavidin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
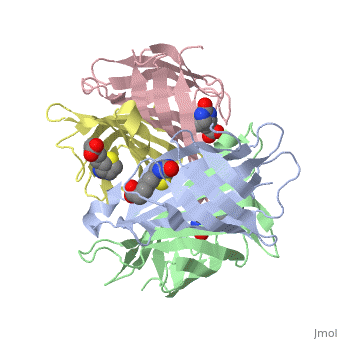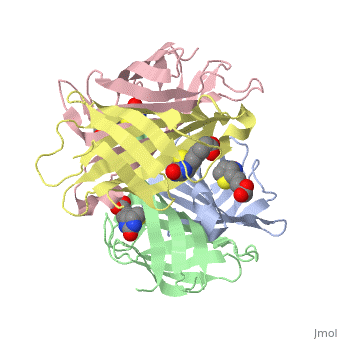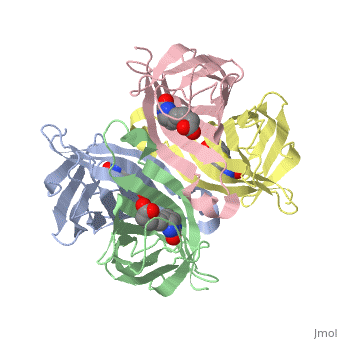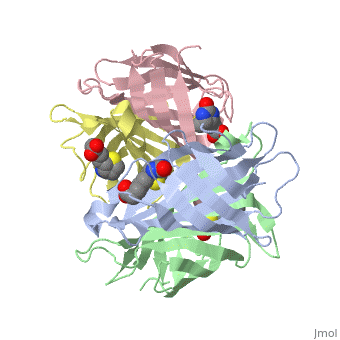
In the [http://www.umas.edu/schiffman Schiffman Lab], we fabricate many types of soft materials including electrospun nanofibers, hydrogels, and nanoparticles. In order to tailor each material for wound management and health care applications, it is necessary to functionalize each material with polymers, enzymes, growth factors or antibiotics. To attach such molecules, we attach streptavidin to our material and take advantage of the streptavidin/biotin bond to add agents such as biotinylated antibodies. Biotin can attach to any of the four subunits of streptavidin. The <scene name='56/567309/F/1'>disappearance</scene> of biotin in the complex shows where biotin can bind to streptavidin. The <scene name='56/567309/Residues_binding/1'>tryptophan residues</scene> comprise part of the biotin-binding site of the streptavidin molecule. | In the [http://www.umas.edu/schiffman Schiffman Lab], we fabricate many types of soft materials including electrospun nanofibers, hydrogels, and nanoparticles. In order to tailor each material for wound management and health care applications, it is necessary to functionalize each material with polymers, enzymes, growth factors or antibiotics. To attach such molecules, we attach streptavidin to our material and take advantage of the streptavidin/biotin bond to add agents such as biotinylated antibodies. Biotin can attach to any of the four subunits of streptavidin. The <scene name='56/567309/F/1'>disappearance</scene> of biotin in the complex shows where biotin can bind to streptavidin. The <scene name='56/567309/Residues_binding/1'>tryptophan residues</scene> comprise part of the biotin-binding site of the streptavidin molecule. | ||
In addition to using Streptavidin for attaching antibiotics, we have also start to use Streptavidin to attach Quantum Dots to materials as new sensing devices. These devices should be utilized to determine the presence of specific markers for a variety of applications including the medical field and water purification. | |||
</StructureSection> | |||
Latest revision as of 21:12, 19 November 2014
The Use of Streptavidin/Biotin Complex to Functionalize MaterialsThe Use of Streptavidin/Biotin Complex to Functionalize Materials
Streptavidin is a protein best known for its ability to bind to biotin creating the strongest non-covalent interaction in nature.
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
In the Schiffman Lab, we fabricate many types of soft materials including electrospun nanofibers, hydrogels, and nanoparticles. In order to tailor each material for wound management and health care applications, it is necessary to functionalize each material with polymers, enzymes, growth factors or antibiotics. To attach such molecules, we attach streptavidin to our material and take advantage of the streptavidin/biotin bond to add agents such as biotinylated antibodies. Biotin can attach to any of the four subunits of streptavidin. The of biotin in the complex shows where biotin can bind to streptavidin. The comprise part of the biotin-binding site of the streptavidin molecule. In addition to using Streptavidin for attaching antibiotics, we have also start to use Streptavidin to attach Quantum Dots to materials as new sensing devices. These devices should be utilized to determine the presence of specific markers for a variety of applications including the medical field and water purification.
|
| ||||||||||