2q0d: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
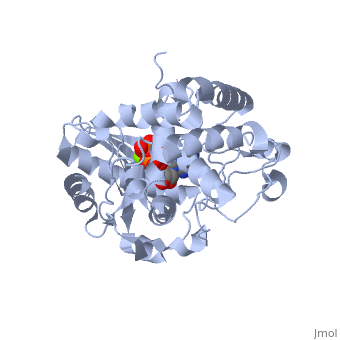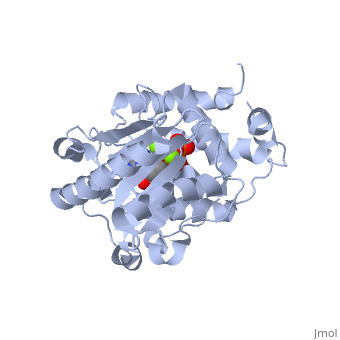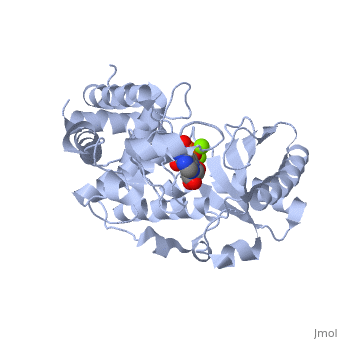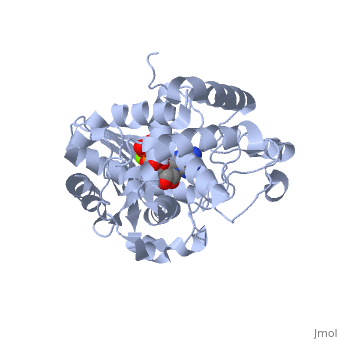
==Terminal uridylyl transferase 4 from Trypanosoma brucei with bound ATP== | |||
=== | <StructureSection load='2q0d' size='340' side='right' caption='[[2q0d]], [[Resolution|resolution]] 2.00Å' scene=''> | ||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[2q0d]] is a 2 chain structure with sequence from [http://en.wikipedia.org/wiki/Trypanosoma_brucei Trypanosoma brucei]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2Q0D OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2Q0D FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ATP:ADENOSINE-5-TRIPHOSPHATE'>ATP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene><br> | |||
<tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2ikf|2ikf]], [[2nom|2nom]]</td></tr> | |||
<tr><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">TUT4 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=5691 Trypanosoma brucei])</td></tr> | |||
<tr><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/RNA_uridylyltransferase RNA uridylyltransferase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.7.7.52 2.7.7.52] </span></td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2q0d FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2q0d OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2q0d RCSB], [http://www.ebi.ac.uk/pdbsum/2q0d PDBsum]</span></td></tr> | |||
<table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/q0/2q0d_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Terminal RNA uridylyltransferases (TUTases) catalyze template-independent UMP addition to the 3' hydroxyl of RNA. TUTases belong to the DNA polymerase beta superfamily of nucleotidyltransferases that share a conserved catalytic domain bearing three metal-binding carboxylate residues. We have previously determined crystal structures of the UTP-bound and apo forms of the minimal trypanosomal TUTase, TbTUT4, which is composed solely of the N-terminal catalytic and C-terminal base-recognition domains. Here we report crystal structures of TbTUT4 with bound CTP, GTP, and ATP, demonstrating nearly perfect superposition of the triphosphate moieties with that of the UTP substrate. Consequently, at physiological nucleoside 5'-triphosphate concentrations, the protein-uracil base interactions alone are not sufficient to confer UTP selectivity. To resolve this ambiguity, we determined the crystal structure of a prereaction ternary complex composed of UTP, TbTUT4, and UMP, which mimics an RNA substrate, and the postreaction complex of TbTUT4 with UpU dinucleotide. The UMP pyrimidine ring stacks against the uracil base of the bound UTP, which on its other face also stacks with an essential tyrosine. In contrast, the different orientation of the purine bases observed in cocrystals with ATP and GTP prevents this triple stacking, precluding productive binding of the RNA. The 3' hydroxyl of the bound UMP is poised for in-line nucleophilic attack while contributing to the formation of a binding site for a second catalytic metal ion. We propose a dual role for RNA substrates in TUTase-catalyzed reactions: contribution to selective incorporation of the cognate nucleoside and shaping of the catalytic metal binding site. | |||
Dual role of the RNA substrate in selectivity and catalysis by terminal uridylyl transferases.,Stagno J, Aphasizheva I, Aphasizhev R, Luecke H Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14634-9. Epub 2007 Sep 4. PMID:17785418<ref>PMID:17785418</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
==See Also== | ==See Also== | ||
*[[RNA uridylyltransferase|RNA uridylyltransferase]] | |||
*[[Terminal Uridylyl Transferase|Terminal Uridylyl Transferase]] | *[[Terminal Uridylyl Transferase|Terminal Uridylyl Transferase]] | ||
== References == | |||
== | <references/> | ||
< | __TOC__ | ||
</StructureSection> | |||
[[Category: RNA uridylyltransferase]] | [[Category: RNA uridylyltransferase]] | ||
[[Category: Trypanosoma brucei]] | [[Category: Trypanosoma brucei]] | ||
Revision as of 22:37, 30 September 2014
Terminal uridylyl transferase 4 from Trypanosoma brucei with bound ATPTerminal uridylyl transferase 4 from Trypanosoma brucei with bound ATP
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedTerminal RNA uridylyltransferases (TUTases) catalyze template-independent UMP addition to the 3' hydroxyl of RNA. TUTases belong to the DNA polymerase beta superfamily of nucleotidyltransferases that share a conserved catalytic domain bearing three metal-binding carboxylate residues. We have previously determined crystal structures of the UTP-bound and apo forms of the minimal trypanosomal TUTase, TbTUT4, which is composed solely of the N-terminal catalytic and C-terminal base-recognition domains. Here we report crystal structures of TbTUT4 with bound CTP, GTP, and ATP, demonstrating nearly perfect superposition of the triphosphate moieties with that of the UTP substrate. Consequently, at physiological nucleoside 5'-triphosphate concentrations, the protein-uracil base interactions alone are not sufficient to confer UTP selectivity. To resolve this ambiguity, we determined the crystal structure of a prereaction ternary complex composed of UTP, TbTUT4, and UMP, which mimics an RNA substrate, and the postreaction complex of TbTUT4 with UpU dinucleotide. The UMP pyrimidine ring stacks against the uracil base of the bound UTP, which on its other face also stacks with an essential tyrosine. In contrast, the different orientation of the purine bases observed in cocrystals with ATP and GTP prevents this triple stacking, precluding productive binding of the RNA. The 3' hydroxyl of the bound UMP is poised for in-line nucleophilic attack while contributing to the formation of a binding site for a second catalytic metal ion. We propose a dual role for RNA substrates in TUTase-catalyzed reactions: contribution to selective incorporation of the cognate nucleoside and shaping of the catalytic metal binding site. Dual role of the RNA substrate in selectivity and catalysis by terminal uridylyl transferases.,Stagno J, Aphasizheva I, Aphasizhev R, Luecke H Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14634-9. Epub 2007 Sep 4. PMID:17785418[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences |
| ||||||||||||||||||||||