1uyn: Difference between revisions
m Protected "1uyn" [edit=sysop:move=sysop] |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image: | ==TRANSLOCATOR DOMAIN OF AUTOTRANSPORTER NALP FROM NEISSERIA MENINGITIDIS== | ||
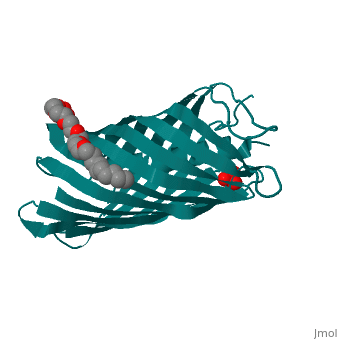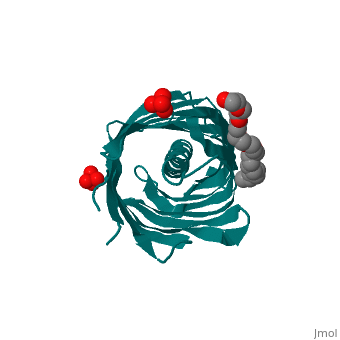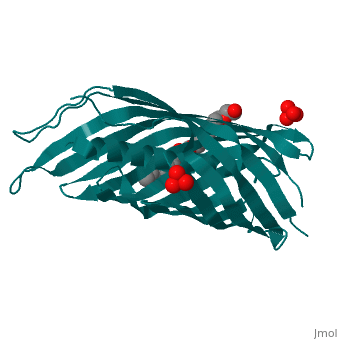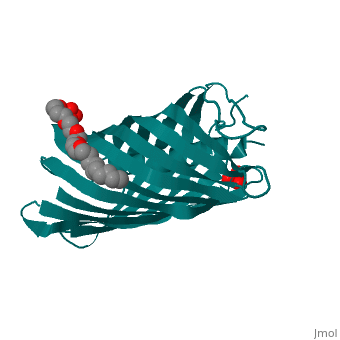
<StructureSection load='1uyn' size='340' side='right' caption='[[1uyn]], [[Resolution|resolution]] 2.60Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1uyn]] is a 1 chain structure with sequence from [http://en.wikipedia.org/wiki/Neisseria_meningitidis Neisseria meningitidis]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1UYN OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1UYN FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=CXE:PENTAETHYLENE+GLYCOL+MONODECYL+ETHER'>CXE</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene><br> | |||
<tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1uyo|1uyo]]</td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1uyn FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1uyn OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1uyn RCSB], [http://www.ebi.ac.uk/pdbsum/1uyn PDBsum]</span></td></tr> | |||
<table> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/uy/1uyn_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
Autotransporters are virulence-related proteins of Gram-negative bacteria that are secreted via an outer-membrane-based C-terminal extension, the translocator domain. This domain supposedly is sufficient for the transport of the N-terminal passenger domain across the outer membrane. We present here the crystal structure of the in vitro-folded translocator domain of the autotransporter NalP from Neisseria meningitidis, which reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A that is filled by an N-terminal alpha-helix. The domain has pore activity in vivo and in vitro. Our data are consistent with the model of passenger-domain transport through the hydrophilic channel within the beta-barrel, and inconsistent with a model for transport through a central channel formed by an oligomer of translocator domains. However, the dimensions of the pore imply translocation of the secreted domain in an unfolded form. An alternative model, possibly covering the transport of folded domains, is that passenger-domain transport involves the Omp85 complex, the machinery required for membrane insertion of outer-membrane proteins, on which autotransporters are dependent. | |||
Structure of the translocator domain of a bacterial autotransporter.,Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P EMBO J. 2004 Mar 24;23(6):1257-66. Epub 2004 Mar 11. PMID:15014442<ref>PMID:15014442</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
==See Also== | |||
*[[Translocator Domain of the Autotransporter NalP within Neisseria meningitidis|Translocator Domain of the Autotransporter NalP within Neisseria meningitidis]] | |||
== | == References == | ||
[[ | <references/> | ||
__TOC__ | |||
== | </StructureSection> | ||
< | |||
[[Category: Neisseria meningitidis]] | [[Category: Neisseria meningitidis]] | ||
[[Category: Feijen, M.]] | [[Category: Feijen, M.]] | ||
Revision as of 00:10, 30 September 2014
TRANSLOCATOR DOMAIN OF AUTOTRANSPORTER NALP FROM NEISSERIA MENINGITIDISTRANSLOCATOR DOMAIN OF AUTOTRANSPORTER NALP FROM NEISSERIA MENINGITIDIS
Structural highlights
Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedAutotransporters are virulence-related proteins of Gram-negative bacteria that are secreted via an outer-membrane-based C-terminal extension, the translocator domain. This domain supposedly is sufficient for the transport of the N-terminal passenger domain across the outer membrane. We present here the crystal structure of the in vitro-folded translocator domain of the autotransporter NalP from Neisseria meningitidis, which reveals a 12-stranded beta-barrel with a hydrophilic pore of 10 x 12.5 A that is filled by an N-terminal alpha-helix. The domain has pore activity in vivo and in vitro. Our data are consistent with the model of passenger-domain transport through the hydrophilic channel within the beta-barrel, and inconsistent with a model for transport through a central channel formed by an oligomer of translocator domains. However, the dimensions of the pore imply translocation of the secreted domain in an unfolded form. An alternative model, possibly covering the transport of folded domains, is that passenger-domain transport involves the Omp85 complex, the machinery required for membrane insertion of outer-membrane proteins, on which autotransporters are dependent. Structure of the translocator domain of a bacterial autotransporter.,Oomen CJ, van Ulsen P, van Gelder P, Feijen M, Tommassen J, Gros P EMBO J. 2004 Mar 24;23(6):1257-66. Epub 2004 Mar 11. PMID:15014442[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||||