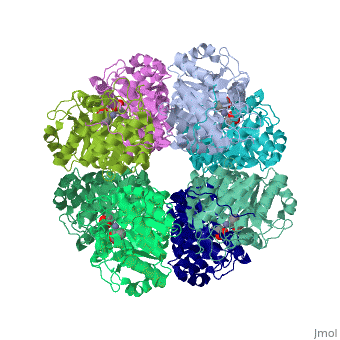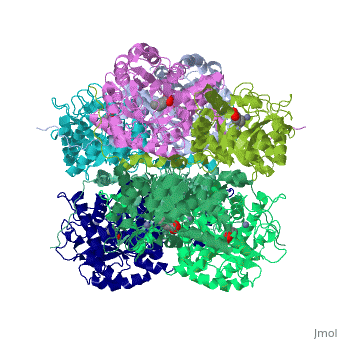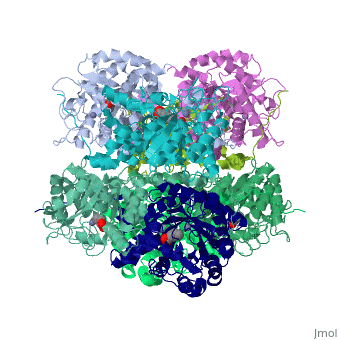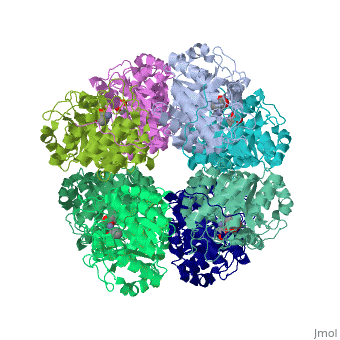1h7n: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1h7n.jpg|left|200px]] | [[Image:1h7n.jpg|left|200px]] | ||
'''SCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTION''' | {{Structure | ||
|PDB= 1h7n |SIZE=350|CAPTION= <scene name='initialview01'>1h7n</scene>, resolution 1.6Å | |||
|SITE= <scene name='pdbsite=AC1:Shf+Binding+Site+For+Chain+A'>AC1</scene> and <scene name='pdbsite=AC2:Zn+Binding+Site+For+Chain+A'>AC2</scene> | |||
|LIGAND= <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene> and <scene name='pdbligand=SHF:LAEVULINIC ACID'>SHF</scene> | |||
|ACTIVITY= [http://en.wikipedia.org/wiki/Porphobilinogen_synthase Porphobilinogen synthase], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=4.2.1.24 4.2.1.24] | |||
|GENE= | |||
}} | |||
'''SCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTION''' | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
1H7N is a [ | 1H7N is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Saccharomyces_cerevisiae Saccharomyces cerevisiae]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1H7N OCA]. | ||
==Reference== | ==Reference== | ||
The x-ray structure of yeast 5-aminolaevulinic acid dehydratase complexed with substrate and three inhibitors., Erskine PT, Newbold R, Brindley AA, Wood SP, Shoolingin-Jordan PM, Warren MJ, Cooper JB, J Mol Biol. 2001 Sep 7;312(1):133-41. PMID:[http:// | The x-ray structure of yeast 5-aminolaevulinic acid dehydratase complexed with substrate and three inhibitors., Erskine PT, Newbold R, Brindley AA, Wood SP, Shoolingin-Jordan PM, Warren MJ, Cooper JB, J Mol Biol. 2001 Sep 7;312(1):133-41. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/11545591 11545591] | ||
[[Category: Porphobilinogen synthase]] | [[Category: Porphobilinogen synthase]] | ||
[[Category: Saccharomyces cerevisiae]] | [[Category: Saccharomyces cerevisiae]] | ||
| Line 29: | Line 38: | ||
[[Category: tim barrel]] | [[Category: tim barrel]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 11:33:23 2008'' | ||
Revision as of 12:33, 20 March 2008
| |||||||
| , resolution 1.6Å | |||||||
|---|---|---|---|---|---|---|---|
| Sites: | and | ||||||
| Ligands: | and | ||||||
| Activity: | Porphobilinogen synthase, with EC number 4.2.1.24 | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
SCHIFF-BASE COMPLEX OF YEAST 5-AMINOLAEVULINIC ACID DEHYDRATASE WITH LAEVULINIC ACID AT 1.6 A RESOLUTION
OverviewOverview
The structures of 5-aminolaevulinic acid dehydratase (ALAD) complexed with substrate (5-aminolaevulinic acid) and three inhibitors: laevulinic acid, succinylacetone and 4-keto-5-aminolaevulinic acid, have been solved at high resolution. The ligands all bind by forming a covalent link with Lys263 at the active site. The structures define the interactions made by one of the two substrate moieties that bind to the enzyme during catalysis. All of the inhibitors induce a significant ordering of the flap covering the active site. Succinylacetone appears to be unique by inducing a number of conformational changes in loops covering the active site, which may be important for understanding the co-operative properties of ALAD enzymes. Succinylacetone is produced in large amounts by patients suffering from the hereditary disease type I tyrosinaemia and its potent inhibition of ALAD also has implications for the pathology of this disease. The most intriguing result is that obtained with 4-keto-5-amino-hexanoic acid, which seems to form a stable carbinolamine intermediate with Lys263. It appears that we have defined the structure of an intermediate of Schiff base formation that the substrate forms upon binding to the P-site of the enzyme.
About this StructureAbout this Structure
1H7N is a Single protein structure of sequence from Saccharomyces cerevisiae. Full crystallographic information is available from OCA.
ReferenceReference
The x-ray structure of yeast 5-aminolaevulinic acid dehydratase complexed with substrate and three inhibitors., Erskine PT, Newbold R, Brindley AA, Wood SP, Shoolingin-Jordan PM, Warren MJ, Cooper JB, J Mol Biol. 2001 Sep 7;312(1):133-41. PMID:11545591
Page seeded by OCA on Thu Mar 20 11:33:23 2008