1gzx: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1gzx.gif|left|200px]] | [[Image:1gzx.gif|left|200px]] | ||
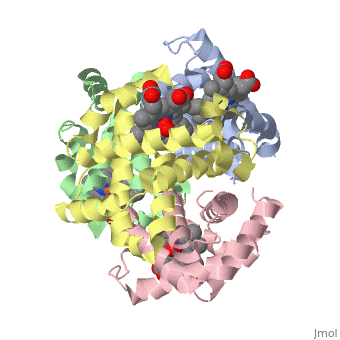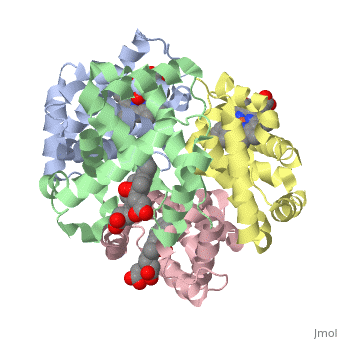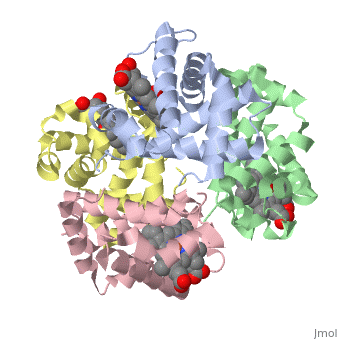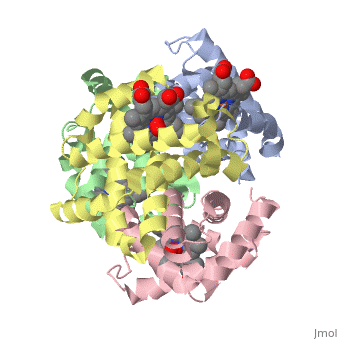
'''OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS''' | {{Structure | ||
|PDB= 1gzx |SIZE=350|CAPTION= <scene name='initialview01'>1gzx</scene>, resolution 2.1Å | |||
|SITE= <scene name='pdbsite=HB1:O2+Binding+Site+For+Chain+D'>HB1</scene> | |||
|LIGAND= <scene name='pdbligand=HEM:PROTOPORPHYRIN+IX+CONTAINING+FE'>HEM</scene> and <scene name='pdbligand=OXY:OXYGEN MOLECULE'>OXY</scene> | |||
|ACTIVITY= | |||
|GENE= | |||
}} | |||
'''OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS''' | |||
==Overview== | ==Overview== | ||
| Line 10: | Line 19: | ||
==About this Structure== | ==About this Structure== | ||
1GZX is a [ | 1GZX is a [[Protein complex]] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1GZX OCA]. | ||
==Reference== | ==Reference== | ||
Crystal structure of T state haemoglobin with oxygen bound at all four haems., Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G, J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:[http:// | Crystal structure of T state haemoglobin with oxygen bound at all four haems., Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G, J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8642597 8642597] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
| Line 28: | Line 37: | ||
[[Category: transport]] | [[Category: transport]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 11:30:17 2008'' | ||
Revision as of 12:30, 20 March 2008
| |||||||
| , resolution 2.1Å | |||||||
|---|---|---|---|---|---|---|---|
| Sites: | |||||||
| Ligands: | and | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
OXY T STATE HAEMOGLOBIN: OXYGEN BOUND AT ALL FOUR HAEMS
OverviewOverview
The cooperative binding of oxygen by haemoglobin results from restraints on ligand binding in the T state. The unfavourable interactions made by the ligands at the haems destabilise the T state and favour the high affinity R state. The T <==> R equilibrium leads, in the presence of a ligand, to a rapid increase in the R state population and therefore generates cooperative binding. There is now considerable understanding of this phenomenon, but the interactions that reduce ligand affinity in the T state have not yet been fully explored, owing to the difficulties in preparing T state haemoglobin crystals in which all the subunits are oxygenated. A protocol has been developed to oxygenate deoxy T state adult human haemoglobin (HbA) crystals in air at 4 C at all four haems without significant loss of crystalline order. The X-ray crystal structure, determined to 2.1 A spacing, shows significant changes in the alpha and beta haem pockets as well as changes at the alpha(1)beta(2) interface in the direction of the R quaternary structure. Most of the shifts and deviations from deoxy T state HbA are similar to, but larger than, those previously observed in the T state met and other partially liganded T state forms. They provide clear evidence of haem-haem interaction in the T state.
DiseaseDisease
Known diseases associated with this structure: Erythremias, alpha- OMIM:[141800], Erythremias, beta- OMIM:[141900], Erythrocytosis OMIM:[141850], HPFH, deletion type OMIM:[141900], Heinz body anemia OMIM:[141850], Heinz body anemias, alpha- OMIM:[141800], Heinz body anemias, beta- OMIM:[141900], Hemoglobin H disease OMIM:[141850], Hypochromic microcytic anemia OMIM:[141850], Methemoglobinemias, alpha- OMIM:[141800], Methemoglobinemias, beta- OMIM:[141900], Sickle cell anemia OMIM:[141900], Thalassemia, alpha- OMIM:[141850], Thalassemia-beta, dominant inclusion-body OMIM:[141900], Thalassemias, alpha- OMIM:[141800], Thalassemias, beta- OMIM:[141900]
About this StructureAbout this Structure
1GZX is a Protein complex structure of sequences from Homo sapiens. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structure of T state haemoglobin with oxygen bound at all four haems., Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G, J Mol Biol. 1996 Mar 8;256(4):775-92. PMID:8642597
Page seeded by OCA on Thu Mar 20 11:30:17 2008