1d5f: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
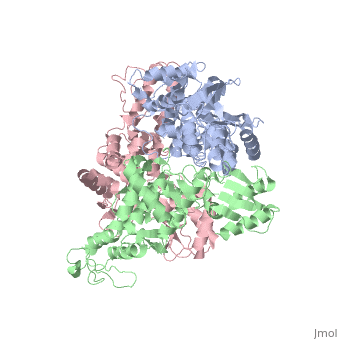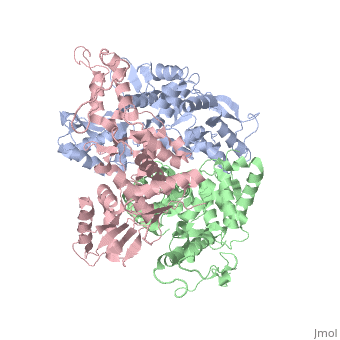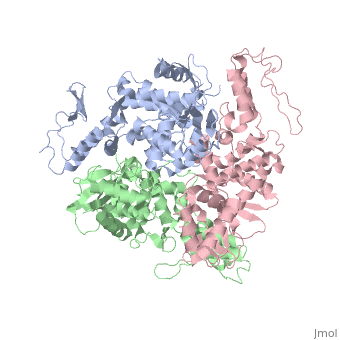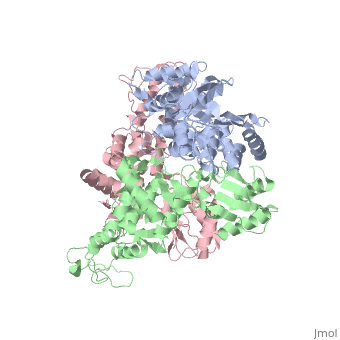
==STRUCTURE OF AN E6AP-UBCH7 COMPLEX: INSIGHTS INTO THE UBIQUITINATION PATHWAY== | |||
<StructureSection load='1d5f' size='340' side='right' caption='[[1d5f]], [[Resolution|resolution]] 2.80Å' scene=''> | |||
== Structural highlights == | |||
<table><tr><td colspan='2'>[[1d5f]] is a 3 chain structure with sequence from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1D5F OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1D5F FirstGlance]. <br> | |||
</td></tr><tr><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1ubq|1ubq]]</td></tr> | |||
<tr><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1d5f FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1d5f OCA], [http://www.rcsb.org/pdb/explore.do?structureId=1d5f RCSB], [http://www.ebi.ac.uk/pdbsum/1d5f PDBsum]</span></td></tr> | |||
<table> | |||
== Disease == | |||
[[http://www.uniprot.org/uniprot/UBE3A_HUMAN UBE3A_HUMAN]] Defects in UBE3A are a cause of Angelman syndrome (AS) [MIM:[http://omim.org/entry/105830 105830]]; also known as 'happy puppet syndrome'. AS is characterized by features of severe motor and intellectual retardation, microcephaly, ataxia, frequent jerky limb movements and flapping of the arms and hands, hypotonia, hyperactivity, hypopigmentation, seizures, absence of speech, frequent smiling and episodes of paroxysmal laughter, and an unusual facies characterized by macrostomia, a large mandible and open-mouthed expression, a great propensity for protruding the tongue ('tongue thrusting'), and an occipital groove.<ref>PMID:10508479</ref> <ref>PMID:9585605</ref> | |||
== Function == | |||
[[http://www.uniprot.org/uniprot/UBE3A_HUMAN UBE3A_HUMAN]] E3 ubiquitin-protein ligase which accepts ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and transfers it to its substrates. Several substrates have been identified including the RAD23A and RAD23B, MCM7 (which is involved in DNA replication), annexin A1, the PML tumor suppressor, and the cell cycle regulator CDKN1B. Catalyzes the high-risk human papilloma virus E6-mediated ubiquitination of p53/TP53, contributing to the neoplastic progression of cells infected by these viruses. Additionally, may function as a cellular quality control ubiquitin ligase by helping the degradation of the cytoplasmic misfolded proteins. Finally, UBE3A also promotes its own degradation in vivo.<ref>PMID:10373495</ref> <ref>PMID:19325566</ref> <ref>PMID:19233847</ref> <ref>PMID:19204938</ref> <ref>PMID:19591933</ref> <ref>PMID:22645313</ref> | |||
== Evolutionary Conservation == | |||
[[Image:Consurf_key_small.gif|200px|right]] | |||
Check<jmol> | |||
<jmolCheckbox> | |||
<scriptWhenChecked>select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/d5/1d5f_consurf.spt"</scriptWhenChecked> | |||
<scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | |||
<text>to colour the structure by Evolutionary Conservation</text> | |||
</jmolCheckbox> | |||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/chain_selection.php?pdb_ID=2ata ConSurf]. | |||
<div style="clear:both"></div> | |||
<div style="background-color:#fffaf0;"> | |||
== Publication Abstract from PubMed == | |||
The E6AP ubiquitin-protein ligase (E3) mediates the human papillomavirus-induced degradation of the p53 tumor suppressor in cervical cancer and is mutated in Angelman syndrome, a neurological disorder. The crystal structure of the catalytic hect domain of E6AP reveals a bilobal structure with a broad catalytic cleft at the junction of the two lobes. The cleft consists of conserved residues whose mutation interferes with ubiquitin-thioester bond formation and is the site of Angelman syndrome mutations. The crystal structure of the E6AP hect domain bound to the UbcH7 ubiquitin-conjugating enzyme (E2) reveals the determinants of E2-E3 specificity and provides insights into the transfer of ubiquitin from the E2 to the E3. | |||
Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade.,Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP Science. 1999 Nov 12;286(5443):1321-6. PMID:10558980<ref>PMID:10558980</ref> | |||
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | |||
</div> | |||
== | ==See Also== | ||
[[ | *[[Ubiquitin protein ligase|Ubiquitin protein ligase]] | ||
== References == | |||
== | <references/> | ||
__TOC__ | |||
</StructureSection> | |||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Beaudenon, S.]] | [[Category: Beaudenon, S.]] | ||
Revision as of 18:14, 29 September 2014
STRUCTURE OF AN E6AP-UBCH7 COMPLEX: INSIGHTS INTO THE UBIQUITINATION PATHWAYSTRUCTURE OF AN E6AP-UBCH7 COMPLEX: INSIGHTS INTO THE UBIQUITINATION PATHWAY
Structural highlights
Disease[UBE3A_HUMAN] Defects in UBE3A are a cause of Angelman syndrome (AS) [MIM:105830]; also known as 'happy puppet syndrome'. AS is characterized by features of severe motor and intellectual retardation, microcephaly, ataxia, frequent jerky limb movements and flapping of the arms and hands, hypotonia, hyperactivity, hypopigmentation, seizures, absence of speech, frequent smiling and episodes of paroxysmal laughter, and an unusual facies characterized by macrostomia, a large mandible and open-mouthed expression, a great propensity for protruding the tongue ('tongue thrusting'), and an occipital groove.[1] [2] Function[UBE3A_HUMAN] E3 ubiquitin-protein ligase which accepts ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and transfers it to its substrates. Several substrates have been identified including the RAD23A and RAD23B, MCM7 (which is involved in DNA replication), annexin A1, the PML tumor suppressor, and the cell cycle regulator CDKN1B. Catalyzes the high-risk human papilloma virus E6-mediated ubiquitination of p53/TP53, contributing to the neoplastic progression of cells infected by these viruses. Additionally, may function as a cellular quality control ubiquitin ligase by helping the degradation of the cytoplasmic misfolded proteins. Finally, UBE3A also promotes its own degradation in vivo.[3] [4] [5] [6] [7] [8] Evolutionary Conservation Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedThe E6AP ubiquitin-protein ligase (E3) mediates the human papillomavirus-induced degradation of the p53 tumor suppressor in cervical cancer and is mutated in Angelman syndrome, a neurological disorder. The crystal structure of the catalytic hect domain of E6AP reveals a bilobal structure with a broad catalytic cleft at the junction of the two lobes. The cleft consists of conserved residues whose mutation interferes with ubiquitin-thioester bond formation and is the site of Angelman syndrome mutations. The crystal structure of the E6AP hect domain bound to the UbcH7 ubiquitin-conjugating enzyme (E2) reveals the determinants of E2-E3 specificity and provides insights into the transfer of ubiquitin from the E2 to the E3. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade.,Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP Science. 1999 Nov 12;286(5443):1321-6. PMID:10558980[9] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
|
| ||||||||||||||||