1atg: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1atg.gif|left|200px]] | [[Image:1atg.gif|left|200px]] | ||
'''AZOTOBACTER VINELANDII PERIPLASMIC MOLYBDATE-BINDING PROTEIN''' | {{Structure | ||
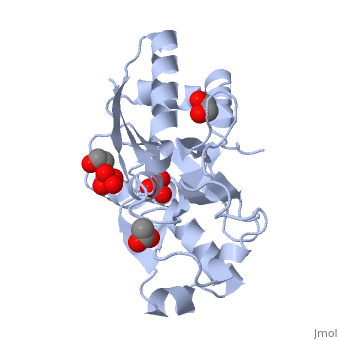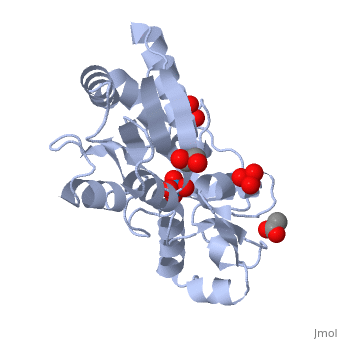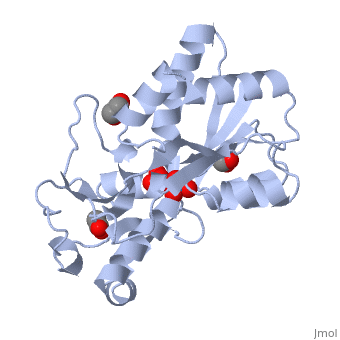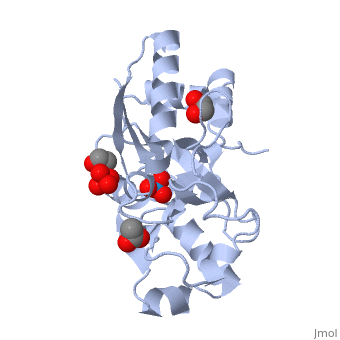
|PDB= 1atg |SIZE=350|CAPTION= <scene name='initialview01'>1atg</scene>, resolution 1.2Å | |||
|SITE= <scene name='pdbsite=MOB:Anion-Binding+Pocket+Located+At+The+Interface+Between+Th+...'>MOB</scene> | |||
|LIGAND= <scene name='pdbligand=WO4:TUNGSTATE(VI)ION'>WO4</scene>, <scene name='pdbligand=ACT:ACETATE+ION'>ACT</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene> and <scene name='pdbligand=EDO:1,2-ETHANEDIOL'>EDO</scene> | |||
|ACTIVITY= | |||
|GENE= | |||
}} | |||
'''AZOTOBACTER VINELANDII PERIPLASMIC MOLYBDATE-BINDING PROTEIN''' | |||
==Overview== | ==Overview== | ||
| Line 7: | Line 16: | ||
==About this Structure== | ==About this Structure== | ||
1ATG is a [ | 1ATG is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Azotobacter_vinelandii Azotobacter vinelandii]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1ATG OCA]. | ||
==Reference== | ==Reference== | ||
Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: the 1.2 A resolution crystal structure of Azotobacter vinelandii ModA., Lawson DM, Williams CE, Mitchenall LA, Pau RN, Structure. 1998 Dec 15;6(12):1529-39. PMID:[http:// | Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: the 1.2 A resolution crystal structure of Azotobacter vinelandii ModA., Lawson DM, Williams CE, Mitchenall LA, Pau RN, Structure. 1998 Dec 15;6(12):1529-39. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/9862806 9862806] | ||
[[Category: Azotobacter vinelandii]] | [[Category: Azotobacter vinelandii]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
| Line 27: | Line 36: | ||
[[Category: tungstate]] | [[Category: tungstate]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Mar 20 10:02:00 2008'' | ||
Revision as of 11:02, 20 March 2008
| |||||||
| , resolution 1.2Å | |||||||
|---|---|---|---|---|---|---|---|
| Sites: | |||||||
| Ligands: | , , and | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
AZOTOBACTER VINELANDII PERIPLASMIC MOLYBDATE-BINDING PROTEIN
OverviewOverview
Background:. Periplasmic receptors constitute a diverse class of binding proteins that differ widely in size, sequence and ligand specificity. Nevertheless, almost all of them display a common beta/alpha folding motif and have similar tertiary structures consisting of two globular domains. The ligand is bound at the bottom of a deep cleft, which lies at the interface between these two domains. The oxyanion-binding proteins are notable in that they can discriminate between very similar ligands. Results:. Azotobacter vinelandii is unusual in that it possesses two periplasmic molybdate-binding proteins. The crystal structure of one of these with bound ligand has been determined at 1.2 A resolution. It superficially resembles the structure of sulphate-binding protein (SBP) from Salmonella typhimurium and uses a similar constellation of hydrogen-bonding interactions to bind its ligand. However, the detailed interactions are distinct from those of SBP and the more closely related molybdate-binding protein of Escherichia coli. Conclusions:. Despite differences in the residues involved in binding, the volumes of the binding pockets in the A. vinelandii and E. coli molybdate-binding proteins are similar and are significantly larger than that of SBP. We conclude that the discrimination between molybdate and sulphate shown by these binding proteins is largely dependent upon small differences in the sizes of these two oxyanions.
About this StructureAbout this Structure
1ATG is a Single protein structure of sequence from Azotobacter vinelandii. Full crystallographic information is available from OCA.
ReferenceReference
Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: the 1.2 A resolution crystal structure of Azotobacter vinelandii ModA., Lawson DM, Williams CE, Mitchenall LA, Pau RN, Structure. 1998 Dec 15;6(12):1529-39. PMID:9862806
Page seeded by OCA on Thu Mar 20 10:02:00 2008