2qc8: Difference between revisions
No edit summary |
No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Glutamine synthetase (GS) catalyzes the ligation of glutamate and ammonia | Glutamine synthetase (GS) catalyzes the ligation of glutamate and ammonia to form glutamine, with concomitant hydrolysis of ATP. In mammals, the activity eliminates cytotoxic ammonia, at the same time converting neurotoxic glutamate to harmless glutamine; there are a number of links between changes in GS activity and neurodegenerative disorders, such as Alzheimer's disease. In plants, because of its importance in the assimilation and re-assimilation of ammonia, the enzyme is a target of some herbicides. GS is also a central component of bacterial nitrogen metabolism and a potential drug target. Previous studies had investigated the structures of bacterial and plant GSs. In the present publication, we report the first structures of mammalian GSs. The apo form of the canine enzyme was solved by molecular replacement and refined at a resolution of 3 A. Two structures of human glutamine synthetase represent complexes with: a) phosphate, ADP, and manganese, and b) a phosphorylated form of the inhibitor methionine sulfoximine, ADP and manganese; these structures were refined to resolutions of 2.05 A and 2.6 A, respectively. Loop movements near the active site generate more closed forms of the eukaryotic enzymes when substrates are bound; the largest changes are associated with the binding of the nucleotide. Comparisons with earlier structures provide a basis for the design of drugs that are specifically directed at either human or bacterial enzymes. The site of binding the amino acid substrate is highly conserved in bacterial and eukaryotic GSs, whereas the nucleotide binding site varies to a much larger degree. Thus, the latter site offers the best target for specific drug design. Differences between mammalian and plant enzymes are much more subtle, suggesting that herbicides targeting GS must be designed with caution. | ||
==Disease== | |||
Known diseases associated with this structure: Glutamine deficiency, congenital OMIM:[[http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=138290 138290]] | |||
==About this Structure== | ==About this Structure== | ||
| Line 10: | Line 13: | ||
==Reference== | ==Reference== | ||
Crystal | Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design., Krajewski WW, Collins R, Holmberg-Schiavone L, Jones TA, Karlberg T, Mowbray SL, J Mol Biol. 2008 Jan 4;375(1):217-28. Epub 2007 Oct 17. PMID:[http://ispc.weizmann.ac.il//pmbin/getpm?pmid=18005987 18005987] | ||
[[Category: Glutamate--ammonia ligase]] | [[Category: Glutamate--ammonia ligase]] | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Arrowsmith, C | [[Category: Arrowsmith, C H.]] | ||
[[Category: Berg, S | [[Category: Berg, S Van Den.]] | ||
[[Category: Berglund, H.]] | [[Category: Berglund, H.]] | ||
[[Category: Busam, R | [[Category: Busam, R D.]] | ||
[[Category: Collins, R.]] | [[Category: Collins, R.]] | ||
[[Category: Dahlgren, L | [[Category: Dahlgren, L G.]] | ||
[[Category: Edwards, A.]] | [[Category: Edwards, A.]] | ||
[[Category: Flodin, S.]] | [[Category: Flodin, S.]] | ||
| Line 36: | Line 39: | ||
[[Category: Nyman, T.]] | [[Category: Nyman, T.]] | ||
[[Category: Persson, C.]] | [[Category: Persson, C.]] | ||
[[Category: SGC, Structural | [[Category: SGC, Structural Genomics Consortium.]] | ||
[[Category: Sagemark, J.]] | [[Category: Sagemark, J.]] | ||
[[Category: Sundstrom, M.]] | [[Category: Sundstrom, M.]] | ||
[[Category: Thorsell, A | [[Category: Thorsell, A G.]] | ||
[[Category: Weigelt, J.]] | [[Category: Weigelt, J.]] | ||
[[Category: ADP]] | [[Category: ADP]] | ||
| Line 52: | Line 55: | ||
[[Category: synthetase]] | [[Category: synthetase]] | ||
''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 18:38:01 2008'' | ||
Revision as of 19:38, 21 February 2008
|
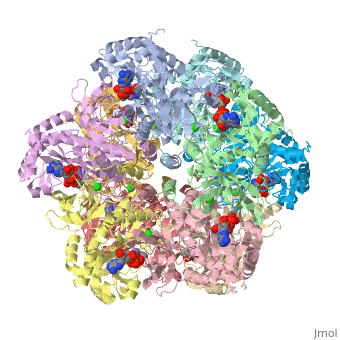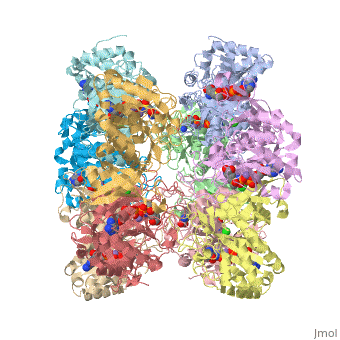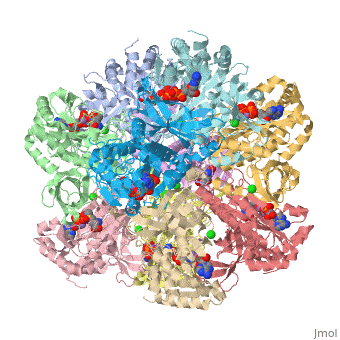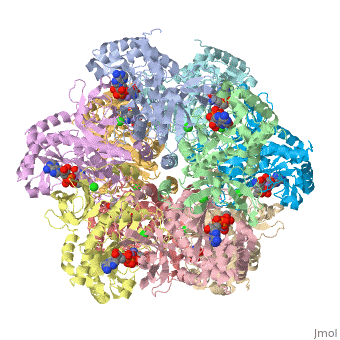
Crystal structure of human glutamine synthetase in complex with ADP and methionine sulfoximine phosphate
OverviewOverview
Glutamine synthetase (GS) catalyzes the ligation of glutamate and ammonia to form glutamine, with concomitant hydrolysis of ATP. In mammals, the activity eliminates cytotoxic ammonia, at the same time converting neurotoxic glutamate to harmless glutamine; there are a number of links between changes in GS activity and neurodegenerative disorders, such as Alzheimer's disease. In plants, because of its importance in the assimilation and re-assimilation of ammonia, the enzyme is a target of some herbicides. GS is also a central component of bacterial nitrogen metabolism and a potential drug target. Previous studies had investigated the structures of bacterial and plant GSs. In the present publication, we report the first structures of mammalian GSs. The apo form of the canine enzyme was solved by molecular replacement and refined at a resolution of 3 A. Two structures of human glutamine synthetase represent complexes with: a) phosphate, ADP, and manganese, and b) a phosphorylated form of the inhibitor methionine sulfoximine, ADP and manganese; these structures were refined to resolutions of 2.05 A and 2.6 A, respectively. Loop movements near the active site generate more closed forms of the eukaryotic enzymes when substrates are bound; the largest changes are associated with the binding of the nucleotide. Comparisons with earlier structures provide a basis for the design of drugs that are specifically directed at either human or bacterial enzymes. The site of binding the amino acid substrate is highly conserved in bacterial and eukaryotic GSs, whereas the nucleotide binding site varies to a much larger degree. Thus, the latter site offers the best target for specific drug design. Differences between mammalian and plant enzymes are much more subtle, suggesting that herbicides targeting GS must be designed with caution.
DiseaseDisease
Known diseases associated with this structure: Glutamine deficiency, congenital OMIM:[138290]
About this StructureAbout this Structure
2QC8 is a Single protein structure of sequence from Homo sapiens with , , and as ligands. Active as Glutamate--ammonia ligase, with EC number 6.3.1.2 Full crystallographic information is available from OCA.
ReferenceReference
Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design., Krajewski WW, Collins R, Holmberg-Schiavone L, Jones TA, Karlberg T, Mowbray SL, J Mol Biol. 2008 Jan 4;375(1):217-28. Epub 2007 Oct 17. PMID:18005987
Page seeded by OCA on Thu Feb 21 18:38:01 2008
Proteopedia Page Contributors and Editors (what is this?)Proteopedia Page Contributors and Editors (what is this?)
OCA- Pages with broken file links
- Glutamate--ammonia ligase
- Homo sapiens
- Single protein
- Arrowsmith, C H.
- Berg, S Van Den.
- Berglund, H.
- Busam, R D.
- Collins, R.
- Dahlgren, L G.
- Edwards, A.
- Flodin, S.
- Flores, A.
- Graslund, S.
- Hammarstrom, M.
- Hogbom, M.
- Holmberg-Schiavone, L.
- Johansson, I.
- Kallas, A.
- Karlberg, T.
- Kotenyova, T.
- Lehtio, L.
- Moche, M.
- Nordlund, P.
- Nyman, T.
- Persson, C.
- SGC, Structural Genomics Consortium.
- Sagemark, J.
- Sundstrom, M.
- Thorsell, A G.
- Weigelt, J.
- ADP
- CL
- MN
- P3S
- Amino-acid biosynthesis
- Ligase
- Sgc
- Structural genomics
- Structural genomics consortium
- Synthetase