Single stranded binding protein: Difference between revisions
No edit summary |
No edit summary |
||
| Line 49: | Line 49: | ||
When His55 is substituted with Leu it decreases binding affinity. All of these residues | When His55 is substituted with Leu it decreases binding affinity. All of these residues | ||
are found in a hydrophobic region, which is suitable for nucleotide base interactions. | are found in a hydrophobic region, which is suitable for nucleotide base interactions. | ||
===SSB-Protein Interactions=== | |||
It is believed that <scene name='56/566528/Gly_15/1'>Gly15</scene> may play an important role in binding the RecA protein. Mutations in Gly15 have | |||
extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, | |||
and a protein n, which is used to help synthesize RNA primers for the lagging strand. | |||
</StructureSection> | </StructureSection> | ||
==See Also== | ==See Also== | ||
Revision as of 20:05, 1 November 2013
Sandbox Single Stranded DNA-Binding Protein (SSB)Sandbox Single Stranded DNA-Binding Protein (SSB)
IntroductionThe single stranded DNA binding protein (SSB) of E. coli plays an important role in three aspects of DNA metabolism – namely in replication, repair and recombination. During DNA replication, SSB molecules bind to the newly separated DNA strands, keeping the strands separated by holding them in place so that each strand can serve as a template [1].
|
| ||||||||||
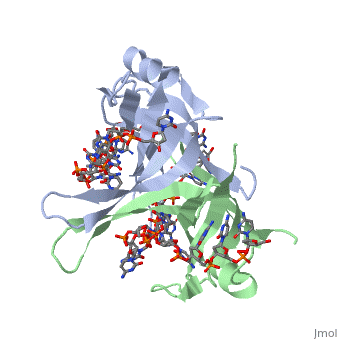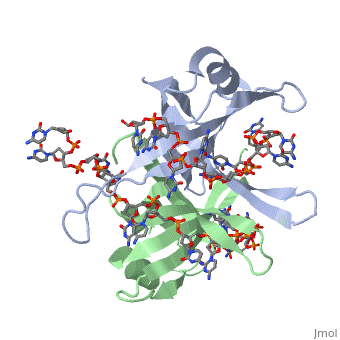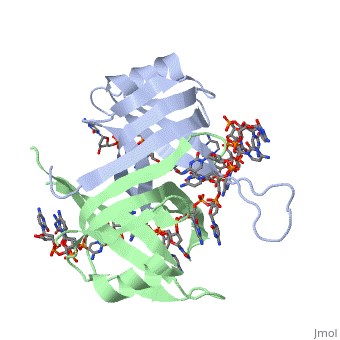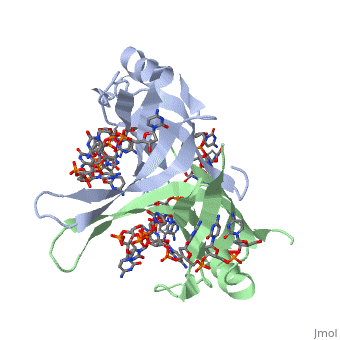
StructureSSB consists is a homotetramer that has a DNA binding domain which binds to a single strand of DNA. The tetramers consist of α-helices, β-sheets, and random coils. Each subunit contains an and several . Binding Interactions in the Active Siteis an important DNA binding site. It has been shown to be the site for cross-linking. Tryptophan and Lysine residues are important in binding as well. Treatments resulting in modification of arginine, cysteine, or tyrosine residues had no effect on binding of SSB to DNA, whereas modification of either lysine residues (with acetic anhydride) or tryptophan residues (with N-bromosuccinimide) led to complete loss of binding activity [2]. The two tryptophan residues involved in DNA binding are Trp40 and Trp54, which was determined by mutagenesis. One more binding site was determined by site-specific mutagenesis. When His55 is substituted with Leu it decreases binding affinity. All of these residues are found in a hydrophobic region, which is suitable for nucleotide base interactions. SSB-Protein InteractionsIt is believed that Gly15 may play an important role in binding the RecA protein. Mutations in Gly15 have extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, and a protein n, which is used to help synthesize RNA primers for the lagging strand. |
| ||||||||||
Binding Interactions between DNA and SSB of E. coliPhe60 is an important DNA binding site. It has been shown to be the site for cross-linking. Tryptophan and Lysine residues are important in binding as well. Treatments resulting in modification of arginine, cysteine, or tyrosine residues had no effect on binding of SSB to DNA, whereas modification of either lysine residues (with acetic anhydride) or tryptophan residues (with N-bromosuccinimide) led to complete loss of binding activity [3]. The two tryptophan residues involved in DNA binding are Trp40 and Trp54, which was determined by mutagenesis. One more binding site was determined by site-specific mutagenesis. When His55 is substituted with Leu it decreases binding affinity. All of these residues are found in a hydrophobic region, which is suitable for nucleotide base interactions. SSB-Protein InteractionsIt is believed that may play an important role in binding the RecA protein. Mutations in Gly15 have extreme effects on recombinational repair. SSB has also been thought to bind with exonuclease I, DNA polymerase II, and a protein n, which is used to help synthesize RNA primers for the lagging strand.
|
| ||||||||||
See AlsoSee Also
ReferencesReferences
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220
- ↑ Meyer RR, Laine PS. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342-80. PMID:2087220