Single stranded binding protein: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
The single-stranded DNA-binding protein (SSB) of Escherichia coli is involved in all aspects of DNA metabolism: replication, repair, and recombination. In solution, the protein exists as a homotetramer of 18,843-kilodalton subunits. As it binds tightly and cooperatively to single-stranded DNA, it has become a prototypic model protein for studying protein-nucleic acid interactions. The sequences of the gene and protein are known, and the functional domains of subunit interaction, DNA binding, and protein-protein interactions have been probed by structure-function analyses of various mutations. The ssb gene has three promoters, one of which is inducible because it lies only two nucleotides from the LexA-binding site of the adjacent uvrA gene. Induction of the SOS response, however, does not lead to significant increases in SSB levels. The binding protein has several functions in DNA replication, including enhancement of helix destabilization by DNA helicases, prevention of reannealing of the single strands and protection from nuclease digestion, organization and stabilization of replication origins, primosome assembly, priming specificity, enhancement of replication fidelity, enhancement of polymerase processivity, and promotion of polymerase binding to the template. E. coli SSB is required for methyl-directed mismatch repair, induction of the SOS response, and recombinational repair. During recombination, SSB interacts with the RecBCD enzyme to find Chi sites, promotes binding of RecA protein, and promotes strand uptake. | The single-stranded DNA-binding protein (SSB) of Escherichia coli is involved in all aspects of DNA metabolism: replication, repair, and recombination. | ||
In solution, the protein exists as a homotetramer of 18,843-kilodalton subunits. As it binds tightly and cooperatively to single-stranded DNA, it has become | |||
a prototypic model protein for studying protein-nucleic acid interactions. The sequences of the gene and protein are known, and the functional domains of | |||
subunit interaction, DNA binding, and protein-protein interactions have been probed by structure-function analyses of various mutations. The ssb gene has | |||
three promoters, one of which is inducible because it lies only two nucleotides from the LexA-binding site of the adjacent uvrA gene. Induction of the SOS | |||
response, however, does not lead to significant increases in SSB levels. The binding protein has several functions in DNA replication, including enhancement | |||
of helix destabilization by DNA helicases, prevention of reannealing of the single strands and protection from nuclease digestion, organization and stabilization | |||
of replication origins, primosome assembly, priming specificity, enhancement of replication fidelity, enhancement of polymerase processivity, and promotion | |||
of polymerase binding to the template. E. coli SSB is required for methyl-directed mismatch repair, induction of the SOS response, and recombinational repair. | |||
During recombination, SSB interacts with the RecBCD enzyme to find Chi sites, promotes binding of RecA protein, and promotes strand uptake. | |||
Copies of SSB bind to the unwound DNA strands, keeping the strands separated so that both strands can serve as templates. | Copies of SSB bind to the unwound DNA strands, keeping the strands separated so that both strands can serve as templates. | ||
| Line 13: | Line 22: | ||
==Binding Interactions in the Active Site== | ==Binding Interactions in the Active Site== | ||
==See Also== | ==See Also== | ||
Revision as of 18:55, 30 October 2013
Sandbox Single Stranded DNA-Binding Protein (SSB)Sandbox Single Stranded DNA-Binding Protein (SSB)
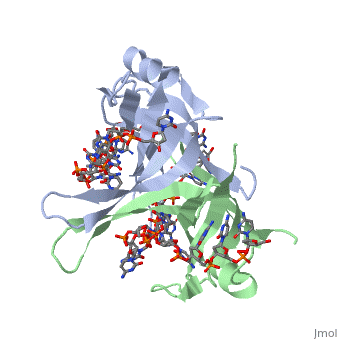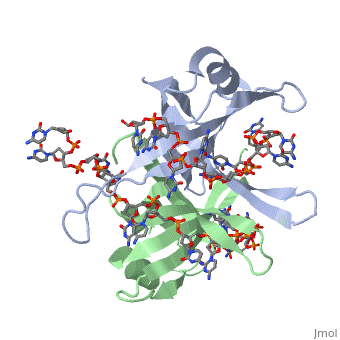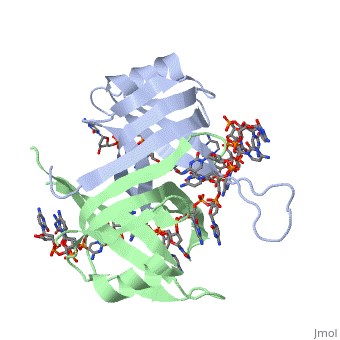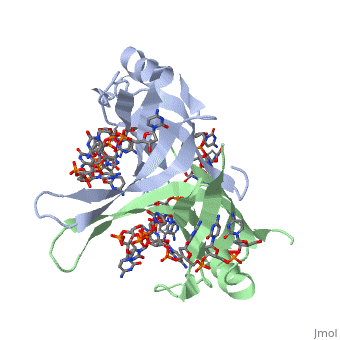
IntroductionThe single-stranded DNA-binding protein (SSB) of Escherichia coli is involved in all aspects of DNA metabolism: replication, repair, and recombination. In solution, the protein exists as a homotetramer of 18,843-kilodalton subunits. As it binds tightly and cooperatively to single-stranded DNA, it has become a prototypic model protein for studying protein-nucleic acid interactions. The sequences of the gene and protein are known, and the functional domains of subunit interaction, DNA binding, and protein-protein interactions have been probed by structure-function analyses of various mutations. The ssb gene has three promoters, one of which is inducible because it lies only two nucleotides from the LexA-binding site of the adjacent uvrA gene. Induction of the SOS response, however, does not lead to significant increases in SSB levels. The binding protein has several functions in DNA replication, including enhancement of helix destabilization by DNA helicases, prevention of reannealing of the single strands and protection from nuclease digestion, organization and stabilization of replication origins, primosome assembly, priming specificity, enhancement of replication fidelity, enhancement of polymerase processivity, and promotion of polymerase binding to the template. E. coli SSB is required for methyl-directed mismatch repair, induction of the SOS response, and recombinational repair. During recombination, SSB interacts with the RecBCD enzyme to find Chi sites, promotes binding of RecA protein, and promotes strand uptake. Copies of SSB bind to the unwound DNA strands, keeping the strands separated so that both strands can serve as templates. StructureThe structure of SSB consists of a homotetramer that has a DNA binding domain that binds to a single strand of DNA. Binding Interactions in the Active SiteSee AlsoReferences
PMID: 2087220
|
| ||||||||||