1e9z: Difference between revisions
No edit summary |
No edit summary |
||
| Line 23: | Line 23: | ||
[[Category: urease]] | [[Category: urease]] | ||
''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on Tue Oct 30 | ''Page seeded by [http://ispc.weizmann.ac.il/oca OCA ] on Tue Oct 30 15:09:23 2007'' | ||
Revision as of 16:04, 30 October 2007
|
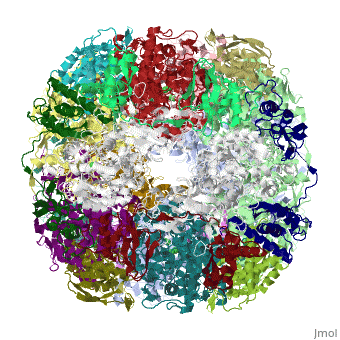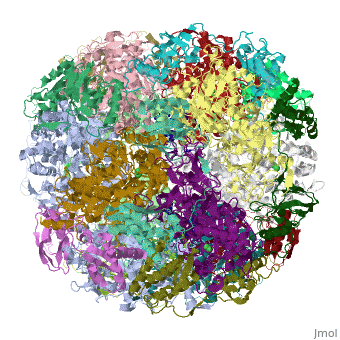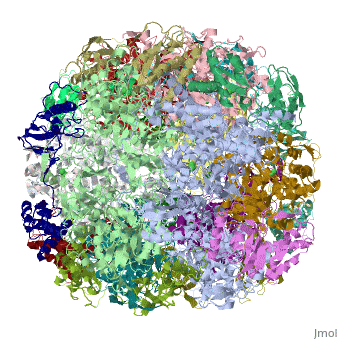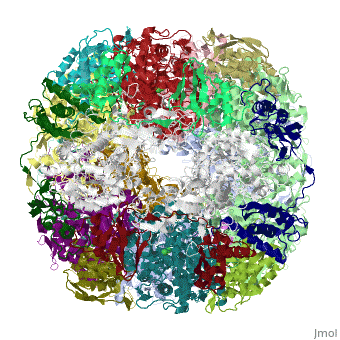
CRYSTAL STRUCTURE OF HELICOBACTER PYLORI UREASE
OverviewOverview
Helicobacter pylori, an etiologic agent in a variety of gastroduodenal, diseases, produces a large amount of urease, which is believed to, neutralize gastric acid by producing ammonia for the survival of the, bacteria. Up to 30% of the enzyme associates with the surface of intact, cells upon lysis of neighboring bacteria. The role of the enzyme at the, extracellular location has been a subject of controversy because the, purified enzyme is irreversibly inactivated below pH 5. We have determined, the crystal structure of H. pylori urease, which has a 1.1 MDa spherical, assembly of 12 catalytic units with an outer diameter of approximately 160, A. Under physiologically relevant conditions, the activity of the enzyme, remains unaffected down to pH 3. Activity assays under different, ... [(full description)]
About this StructureAbout this Structure
1E9Z is a [Protein complex] structure of sequences from [Helicobacter pylori] with NI as [ligand]. Active as [Urease], with EC number [3.5.1.5]. Structure known Active Site: NI1. Full crystallographic information is available from [OCA].
ReferenceReference
Supramolecular assembly and acid resistance of Helicobacter pylori urease., Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH, Nat Struct Biol. 2001 Jun;8(6):505-9. PMID:11373617
Page seeded by OCA on Tue Oct 30 15:09:23 2007