CcNiR: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
== Your Heading Here ('ccNiR') == | == Your Heading Here ('ccNiR') == | ||
<StructureSection load='1oah' size='500' side='left' caption='CYTOCHROME C NITRITE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS ATCC 27774 (PDB entry [[1oah]])' scene=''> | <StructureSection load='1oah' size='500' side='left' caption='CYTOCHROME C NITRITE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS ATCC 27774 (PDB entry [[1oah]])' scene=''> | ||
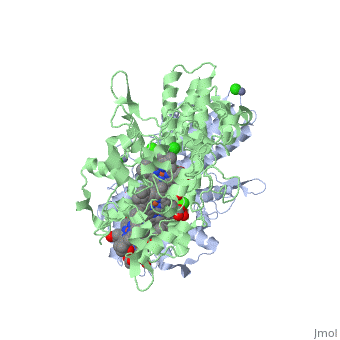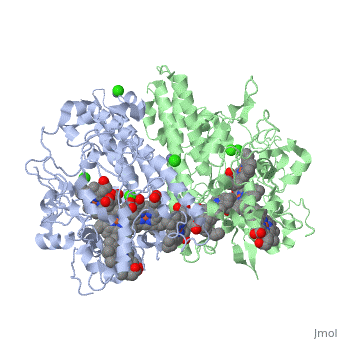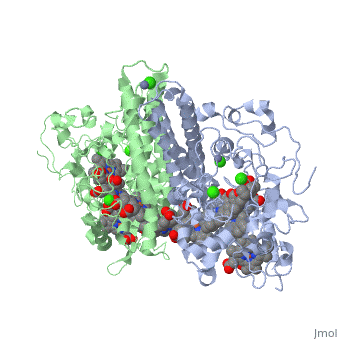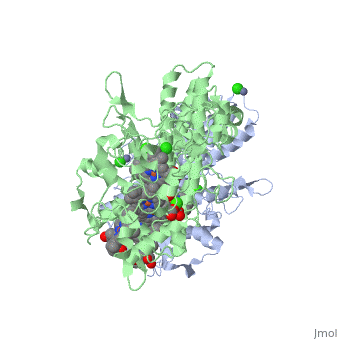
Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated | Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated to perform the reduction of nitrite to ammonium in a six-electron transfer reaction/ <scene name='CcNiR/Hemes/1'>hemes</scene> <ref>pmid 14511372</ref> <ref>pmid 12618432</ref> <ref>pmid 8798514</ref> <ref>pmid 17207484</ref> <ref>pmid 20689707</ref> <ref>pmid 17207484</ref> | ||
Revision as of 14:10, 6 September 2012
Your Heading Here ('ccNiR')Your Heading Here ('ccNiR')
| |||||||||||
Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction. In the sulfate reducing bacterium Desulfovibrio desulfuricans ATCC 27774, the enzyme is purified as a NrfA2NrfH complex that houses 14 hemes.
The catalytic reaction occurs at a high-spin (5-coordinated) heme that is located at the pentahemic subunit NrfA which is strongly bound to its physiological electron donor, the smaller hydrophobic polypeptide tetrahemic NrfH, composed of 4 c-types hemes; in vitro, the protein complexes associate each other forming huge aggregates (min. 890 kDa).
- ↑ Almeida MG, Macieira S, Goncalves LL, Huber R, Cunha CA, Romao MJ, Costa C, Lampreia J, Moura JJ, Moura I. The isolation and characterization of cytochrome c nitrite reductase subunits (NrfA and NrfH) from Desulfovibrio desulfuricans ATCC 27774. Re-evaluation of the spectroscopic data and redox properties. Eur J Biochem. 2003 Oct;270(19):3904-15. PMID:14511372
- ↑ Cunha CA, Macieira S, Dias JM, Almeida G, Goncalves LL, Costa C, Lampreia J, Huber R, Moura JJ, Moura I, Romao MJ. Cytochrome c nitrite reductase from Desulfovibrio desulfuricans ATCC 27774. The relevance of the two calcium sites in the structure of the catalytic subunit (NrfA). J Biol Chem. 2003 May 9;278(19):17455-65. Epub 2003 Mar 4. PMID:12618432 doi:10.1074/jbc.M211777200
- ↑ Costa C, Moura JJ, Moura I, Wang Y, Huynh BH. Redox properties of cytochrome c nitrite reductase from Desulfovibrio desulfuricans ATCC 27774. J Biol Chem. 1996 Sep 20;271(38):23191-6. PMID:8798514
- ↑ Almeida MG, Silveira CM, Guigliarelli B, Bertrand P, Moura JJ, Moura I, Leger C. A needle in a haystack: the active site of the membrane-bound complex cytochrome c nitrite reductase. FEBS Lett. 2007 Jan 23;581(2):284-8. Epub 2006 Dec 19. PMID:17207484 doi:10.1016/j.febslet.2006.12.023
- ↑ Silveira CM, Besson S, Moura I, Moura JJ, Almeida MG. Measuring the cytochrome C nitrite reductase activity-practical considerations on the enzyme assays. Bioinorg Chem Appl. 2010. pii: 634597. Epub 2010 Jun 22. PMID:20689707 doi:10.1155/2010/634597
- ↑ Almeida MG, Silveira CM, Guigliarelli B, Bertrand P, Moura JJ, Moura I, Leger C. A needle in a haystack: the active site of the membrane-bound complex cytochrome c nitrite reductase. FEBS Lett. 2007 Jan 23;581(2):284-8. Epub 2006 Dec 19. PMID:17207484 doi:10.1016/j.febslet.2006.12.023