CcNiR: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
== Your Heading Here ('ccNiR') == | == Your Heading Here ('ccNiR') == | ||
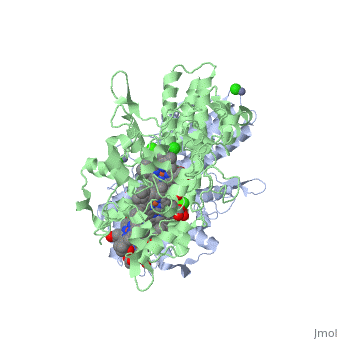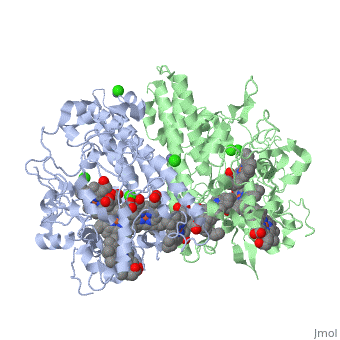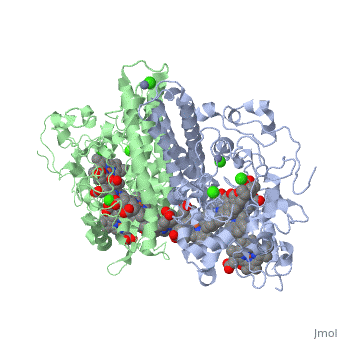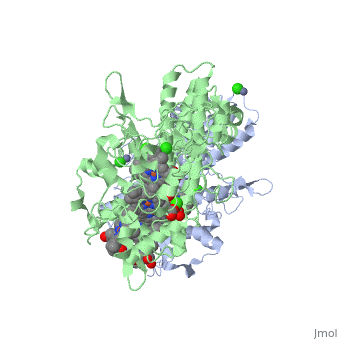
<StructureSection load='1oah' size='500' side='left' caption='CYTOCHROME C NITRITE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS ATCC 27774 (PDB entry [[1oah]])' scene=''> | <StructureSection load='1oah' size='500' side='left' caption='CYTOCHROME C NITRITE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS ATCC 27774 (PDB entry [[1oah]])' scene=''> | ||
Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction <ref>pmid 14511372</ref> | Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction <ref>pmid 14511372</ref> <ref>pmid 8798514</ref> | ||
</StructureSection> | </StructureSection> | ||
| Line 8: | Line 8: | ||
Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction. In the sulfate reducing bacterium Desulfovibrio desulfuricans ATCC 27774, the enzyme is purified as a NrfA2NrfH complex that houses 14 hemes. | Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction. In the sulfate reducing bacterium Desulfovibrio desulfuricans ATCC 27774, the enzyme is purified as a NrfA2NrfH complex that houses 14 hemes. | ||
The catalytic reaction occurs at a high-spin (5-coordinated) heme that is located at | The catalytic reaction occurs at a high-spin (5-coordinated) heme that is located at the pentahemic subunit NrfA which is strongly bound to its physiological electron donor, the smaller hydrophobic polypeptide tetrahemic NrfH, composed of 4 c-types hemes; in vitro, the protein complexes associate each other forming huge aggregates (min. 890 kDa). | ||
<references/> | <references/> | ||
Revision as of 11:26, 6 September 2012
Your Heading Here ('ccNiR')Your Heading Here ('ccNiR')
| |||||||||||
Cytochrome c nitrite reductase is a multicenter enzyme that uses a five-coordinated heme to perform the reduction of nitrite to ammonium in a six-electron transfer reaction. In the sulfate reducing bacterium Desulfovibrio desulfuricans ATCC 27774, the enzyme is purified as a NrfA2NrfH complex that houses 14 hemes.
The catalytic reaction occurs at a high-spin (5-coordinated) heme that is located at the pentahemic subunit NrfA which is strongly bound to its physiological electron donor, the smaller hydrophobic polypeptide tetrahemic NrfH, composed of 4 c-types hemes; in vitro, the protein complexes associate each other forming huge aggregates (min. 890 kDa).
- ↑ Almeida MG, Macieira S, Goncalves LL, Huber R, Cunha CA, Romao MJ, Costa C, Lampreia J, Moura JJ, Moura I. The isolation and characterization of cytochrome c nitrite reductase subunits (NrfA and NrfH) from Desulfovibrio desulfuricans ATCC 27774. Re-evaluation of the spectroscopic data and redox properties. Eur J Biochem. 2003 Oct;270(19):3904-15. PMID:14511372
- ↑ Costa C, Moura JJ, Moura I, Wang Y, Huynh BH. Redox properties of cytochrome c nitrite reductase from Desulfovibrio desulfuricans ATCC 27774. J Biol Chem. 1996 Sep 20;271(38):23191-6. PMID:8798514