G18secL03Tpc4: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
*Primary Structure | *Primary Structure | ||
The ospC gene is located on a 27 kb circular plasmid and encodes a lipoprotein of 22–23 kDa.<ref>PMID:7679385</ref> The protein is initially synthesized with an 18-amino-acid-long signal sequence which is removed during processing and lipidation at the amino proximal Cys residue. OspC proteins are highly polymorphic and this variability extends even to strains collected from a single geographical area.<ref>D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [http://dx.doi.org/DOI:10.1093/emboj/20.5.971]</ref> | The ospC gene is located on a 27 kb circular plasmid and encodes a lipoprotein of 22–23 kDa.<ref>PMID:7679385</ref> The protein is initially synthesized with an 18-amino-acid-long signal sequence which is removed during processing and lipidation at the amino proximal Cys residue. OspC proteins are highly polymorphic and this variability extends even to strains collected from a single geographical area.<ref>D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [http://dx.doi.org/DOI:10.1093/emboj/20.5.971]</ref> | ||
*Secondary Structure | *Secondary Structure | ||
OspC is predominantly α-helical in its secondary structure. β-sheets are also present, but they are rather short and not promininent. | |||
*Tertiary Structure | *Tertiary Structure | ||
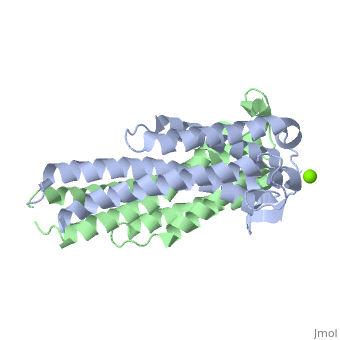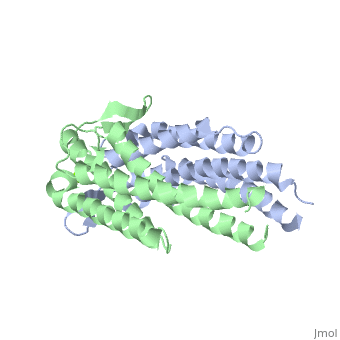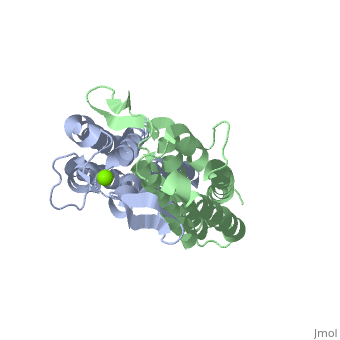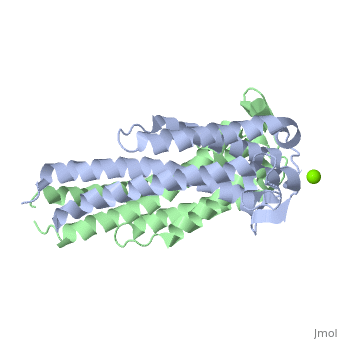
A single OspC subunit is composed of 5 long and 1 short α-helices. Also, 2 short segments of β-sheets are observed near the binding site of the molecule. | |||
*Quaternary Structure | |||
OspC is a dimerized molecule, with 2 identical subunits comprising a dimer. However, for a binding event to occur, 2 dimers are necessary. | |||
=== Main Function === | === Main Function === | ||
Revision as of 03:26, 15 August 2012
|
Outer surface protein C (OspC) of Borrelia burgdorferiOuter surface protein C (OspC) of Borrelia burgdorferi
Outer surface protein C (OspC) is one of the major antigens on the surface of the Lyme disease spirochete, Borrelia burgdorferi, along with other outer surface proteins A and B (OspA and OspB, respectively). It greatly differs from OspA and OspB in both structure and function. The uniqueness of OspC is that it comes into play when the pathogen is being transmitted to humans or other mammals.OspC is critical for survival in or transmission to the tick or mammalian host.[1] OspC is being produced by Borrelia burgdorferi during a very short time interval when infected ticks start feeding, but its synthesis is known to slow down greatly after transmission to a mammalian host. It was demonstrated that those spirochetes that lack OspC are capable to replicate inside and migrate to the salivary glands of the tick vector but do not infect mammals. [2] Without OspC the spirochetes are believed to be unable to adapt to the environment inside the host. Therefore, OspC is believed to determine virulence of the spirochete to mammals, including humans.
Basic Structure DescriptionBasic Structure Description
- Primary Structure
The ospC gene is located on a 27 kb circular plasmid and encodes a lipoprotein of 22–23 kDa.[3] The protein is initially synthesized with an 18-amino-acid-long signal sequence which is removed during processing and lipidation at the amino proximal Cys residue. OspC proteins are highly polymorphic and this variability extends even to strains collected from a single geographical area.[4]
- Secondary Structure
OspC is predominantly α-helical in its secondary structure. β-sheets are also present, but they are rather short and not promininent.
- Tertiary Structure
A single OspC subunit is composed of 5 long and 1 short α-helices. Also, 2 short segments of β-sheets are observed near the binding site of the molecule.
- Quaternary Structure
OspC is a dimerized molecule, with 2 identical subunits comprising a dimer. However, for a binding event to occur, 2 dimers are necessary.
Main FunctionMain Function
Putative Binding SitePutative Binding Site
Role of OspC in Lyme DiseaseRole of OspC in Lyme Disease
OspC-based Vaccine Against Lyme DiseaseOspC-based Vaccine Against Lyme Disease
Main Advantages of Developing OspC-based VaccineMain Advantages of Developing OspC-based Vaccine
Main Problems with Application of OspC-based VaccineMain Problems with Application of OspC-based Vaccine
Notes and References
- ↑ Stewart PE, Thalken R, Bono JL, Rosa P. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol Microbiol. 2001 Feb;39(3):714-21. PMID:11169111
- ↑ D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [1]
- ↑ Marconi RT, Samuels DS, Garon CF. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993 Feb;175(4):926-32. PMID:7679385
- ↑ D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [2]