G18secL03Tpc4: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
<Structure load='1ggq' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | <Structure load='1ggq' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
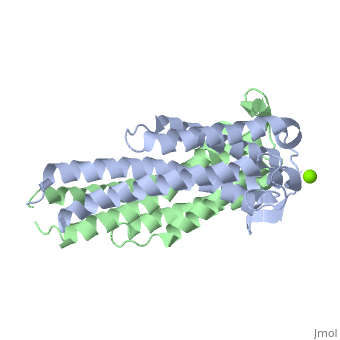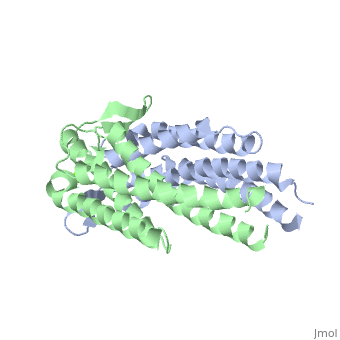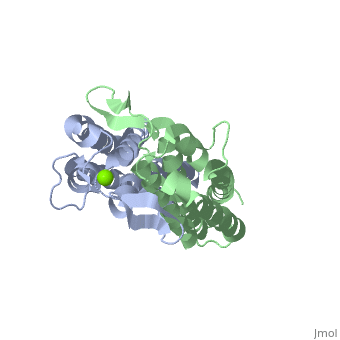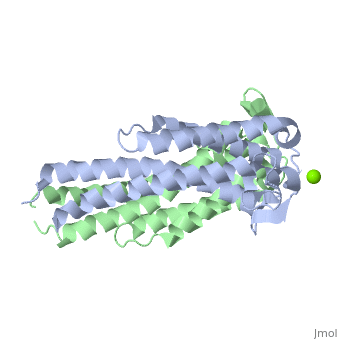
==='''Outer surface protein C (OspC) of ''Borrelia burgdorferi'''=== | ==='''Outer surface protein C (OspC) of ''Borrelia burgdorferi'''=== | ||
Outer surface protein C (OspC) is one of the major antigens on the surface of the Lyme disease spirochete, ''Borrelia burgdorferi'', along with other outer surface proteins A and B (OspA and OspB, respectively). It greatly differs from OspA and OspB in both structure and function. The uniqueness of OspC is that it comes into play when the pathogen is being transmitted to humans or other mammals. OspC is being produced by Borrelia burgdorferi during a very short time interval when infected ticks start feeding, but its synthesis is known to slow down greatly after transmission to a mammalian host. It was demonstrated that those spirochetes that lack OspC are capable to replicate inside and migrate to the salivary glands of the tick vector but do not infect mammals. <ref>D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [http://dx.doi.org/DOI:10.1093/emboj/20.5.971]</ref> Therefore, OspC is believed to determine virulence of the spirochete to mammals, including humans. | Outer surface protein C (OspC) is one of the major antigens on the surface of the Lyme disease spirochete, ''Borrelia burgdorferi'', along with other outer surface proteins A and B (OspA and OspB, respectively). It greatly differs from OspA and OspB in both structure and function. The uniqueness of OspC is that it comes into play when the pathogen is being transmitted to humans or other mammals. OspC is being produced by Borrelia burgdorferi during a very short time interval when infected ticks start feeding, but its synthesis is known to slow down greatly after transmission to a mammalian host. It was demonstrated that those spirochetes that lack OspC are capable to replicate inside and migrate to the salivary glands of the tick vector but do not infect mammals. <ref>D. Kumaran1, S. Eswaramoorthy1, B.J. Luft2, S. Koide3, J.J. Dunn1, C.L. Lawson1,4 and S. Swaminathan1. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi.The EMBO Journal (2001) 20, 971 - 978 [http://dx.doi.org/DOI:10.1093/emboj/20.5.971]</ref> Without OspC the spirochetes are believed to be unable to adapt to the environment inside the host. Therefore, OspC is believed to determine virulence of the spirochete to mammals, including humans. | ||
Revision as of 02:10, 15 August 2012
|
Outer surface protein C (OspC) of Borrelia burgdorferiOuter surface protein C (OspC) of Borrelia burgdorferi
Outer surface protein C (OspC) is one of the major antigens on the surface of the Lyme disease spirochete, Borrelia burgdorferi, along with other outer surface proteins A and B (OspA and OspB, respectively). It greatly differs from OspA and OspB in both structure and function. The uniqueness of OspC is that it comes into play when the pathogen is being transmitted to humans or other mammals. OspC is being produced by Borrelia burgdorferi during a very short time interval when infected ticks start feeding, but its synthesis is known to slow down greatly after transmission to a mammalian host. It was demonstrated that those spirochetes that lack OspC are capable to replicate inside and migrate to the salivary glands of the tick vector but do not infect mammals. [1] Without OspC the spirochetes are believed to be unable to adapt to the environment inside the host. Therefore, OspC is believed to determine virulence of the spirochete to mammals, including humans.