1emv: Difference between revisions
New page: left|200px<br /><applet load="1emv" size="450" color="white" frame="true" align="right" spinBox="true" caption="1emv, resolution 1.7Å" /> '''CRYSTAL STRUCTURE OF ... |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1emv.gif|left|200px]]<br /><applet load="1emv" size=" | [[Image:1emv.gif|left|200px]]<br /><applet load="1emv" size="350" color="white" frame="true" align="right" spinBox="true" | ||
caption="1emv, resolution 1.7Å" /> | caption="1emv, resolution 1.7Å" /> | ||
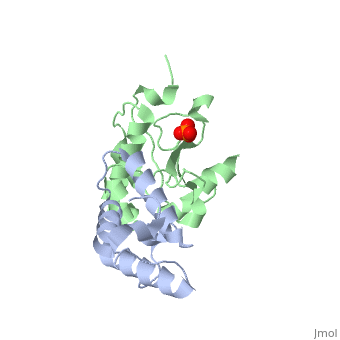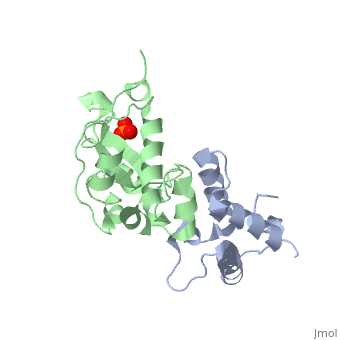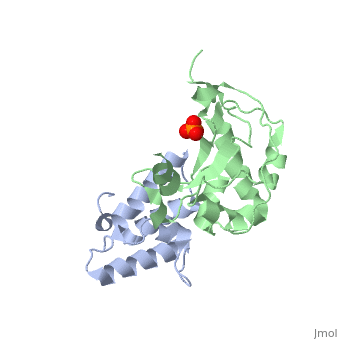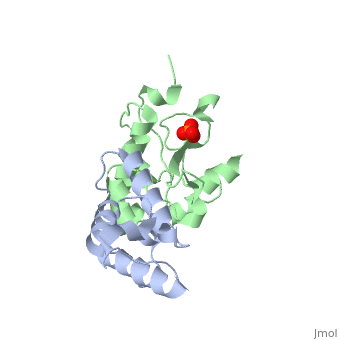
'''CRYSTAL STRUCTURE OF COLICIN E9 DNASE DOMAIN WITH ITS COGNATE IMMUNITY PROTEIN IM9 (1.7 ANGSTROMS)'''<br /> | '''CRYSTAL STRUCTURE OF COLICIN E9 DNASE DOMAIN WITH ITS COGNATE IMMUNITY PROTEIN IM9 (1.7 ANGSTROMS)'''<br /> | ||
==Overview== | ==Overview== | ||
Bacteria producing endonuclease colicins are protected against their | Bacteria producing endonuclease colicins are protected against their cytotoxic activity by virtue of a small immunity protein that binds with high affinity and specificity to inactivate the endonuclease. DNase binding by the immunity protein occurs through a "dual recognition" mechanism in which conserved residues from helix III act as the binding-site anchor, while variable residues from helix II define specificity. We now report the 1.7 A crystal structure of the 24.5 kDa complex formed between the endonuclease domain of colicin E9 and its cognate immunity protein Im9, which provides a molecular rationale for this mechanism. Conserved residues of Im9 form a binding-energy hotspot through a combination of backbone hydrogen bonds to the endonuclease, many via buried solvent molecules, and hydrophobic interactions at the core of the interface, while the specificity-determining residues interact with corresponding specificity side-chains on the enzyme. Comparison between the present structure and that reported recently for the colicin E7 endonuclease domain in complex with Im7 highlights how specificity is achieved by very different interactions in the two complexes, predominantly hydrophobic in nature in the E9-Im9 complex but charged in the E7-Im7 complex. A key feature of both complexes is the contact between a conserved tyrosine residue from the immunity proteins (Im9 Tyr54) with a specificity residue on the endonuclease directing it toward the specificity sites of the immunity protein. Remarkably, this tyrosine residue and its neighbour (Im9 Tyr55) are the pivots of a 19 degrees rigid-body rotation that relates the positions of Im7 and Im9 in the two complexes. This rotation does not affect conserved immunity protein interactions with the endonuclease but results in different regions of the specificity helix being presented to the enzyme. | ||
==About this Structure== | ==About this Structure== | ||
1EMV is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli] with PO4 as [http://en.wikipedia.org/wiki/ligand ligand]. Active as [http://en.wikipedia.org/wiki/Deoxyribonuclease_I Deoxyribonuclease I], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.1.21.1 3.1.21.1] Full crystallographic information is available from [http:// | 1EMV is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli] with <scene name='pdbligand=PO4:'>PO4</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. Active as [http://en.wikipedia.org/wiki/Deoxyribonuclease_I Deoxyribonuclease I], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.1.21.1 3.1.21.1] Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1EMV OCA]. | ||
==Reference== | ==Reference== | ||
| Line 16: | Line 16: | ||
[[Category: James, R.]] | [[Category: James, R.]] | ||
[[Category: Kleanthous, C.]] | [[Category: Kleanthous, C.]] | ||
[[Category: Kuhlmann, U | [[Category: Kuhlmann, U C.]] | ||
[[Category: Moore, G | [[Category: Moore, G M.]] | ||
[[Category: Pommer, A | [[Category: Pommer, A J.]] | ||
[[Category: PO4]] | [[Category: PO4]] | ||
[[Category: protein-protein complex]] | [[Category: protein-protein complex]] | ||
''Page seeded by [http:// | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 12:29:27 2008'' | ||
Revision as of 13:29, 21 February 2008
|
CRYSTAL STRUCTURE OF COLICIN E9 DNASE DOMAIN WITH ITS COGNATE IMMUNITY PROTEIN IM9 (1.7 ANGSTROMS)
OverviewOverview
Bacteria producing endonuclease colicins are protected against their cytotoxic activity by virtue of a small immunity protein that binds with high affinity and specificity to inactivate the endonuclease. DNase binding by the immunity protein occurs through a "dual recognition" mechanism in which conserved residues from helix III act as the binding-site anchor, while variable residues from helix II define specificity. We now report the 1.7 A crystal structure of the 24.5 kDa complex formed between the endonuclease domain of colicin E9 and its cognate immunity protein Im9, which provides a molecular rationale for this mechanism. Conserved residues of Im9 form a binding-energy hotspot through a combination of backbone hydrogen bonds to the endonuclease, many via buried solvent molecules, and hydrophobic interactions at the core of the interface, while the specificity-determining residues interact with corresponding specificity side-chains on the enzyme. Comparison between the present structure and that reported recently for the colicin E7 endonuclease domain in complex with Im7 highlights how specificity is achieved by very different interactions in the two complexes, predominantly hydrophobic in nature in the E9-Im9 complex but charged in the E7-Im7 complex. A key feature of both complexes is the contact between a conserved tyrosine residue from the immunity proteins (Im9 Tyr54) with a specificity residue on the endonuclease directing it toward the specificity sites of the immunity protein. Remarkably, this tyrosine residue and its neighbour (Im9 Tyr55) are the pivots of a 19 degrees rigid-body rotation that relates the positions of Im7 and Im9 in the two complexes. This rotation does not affect conserved immunity protein interactions with the endonuclease but results in different regions of the specificity helix being presented to the enzyme.
About this StructureAbout this Structure
1EMV is a Protein complex structure of sequences from Escherichia coli with as ligand. Active as Deoxyribonuclease I, with EC number 3.1.21.1 Full crystallographic information is available from OCA.
ReferenceReference
Specificity in protein-protein interactions: the structural basis for dual recognition in endonuclease colicin-immunity protein complexes., Kuhlmann UC, Pommer AJ, Moore GR, James R, Kleanthous C, J Mol Biol. 2000 Sep 1;301(5):1163-78. PMID:10966813
Page seeded by OCA on Thu Feb 21 12:29:27 2008