1b3q: Difference between revisions
New page: left|200px<br /><applet load="1b3q" size="450" color="white" frame="true" align="right" spinBox="true" caption="1b3q, resolution 2.6Å" /> '''CRYSTAL STRUCTURE OF ... |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1b3q.gif|left|200px]]<br /><applet load="1b3q" size=" | [[Image:1b3q.gif|left|200px]]<br /><applet load="1b3q" size="350" color="white" frame="true" align="right" spinBox="true" | ||
caption="1b3q, resolution 2.6Å" /> | caption="1b3q, resolution 2.6Å" /> | ||
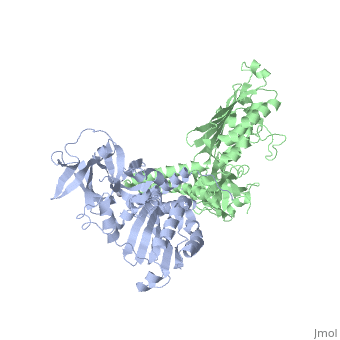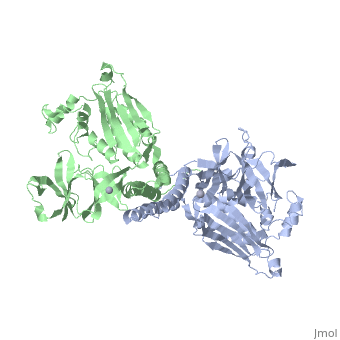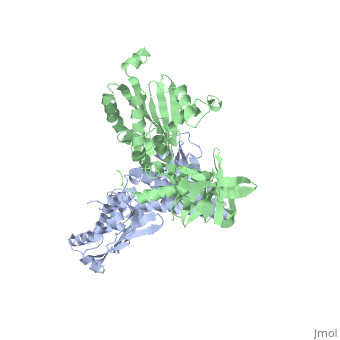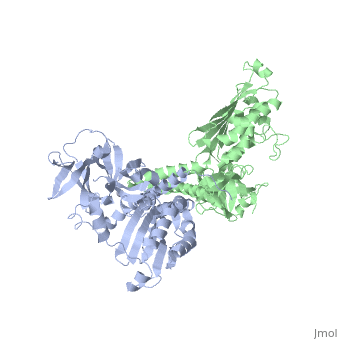
'''CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE'''<br /> | '''CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE'''<br /> | ||
==Overview== | ==Overview== | ||
Histidine kinases allow bacteria, plants, and fungi to sense and respond | Histidine kinases allow bacteria, plants, and fungi to sense and respond to their environment. The 2.6 A resolution crystal structure of Thermotoga maritima CheA (290-671) histidine kinase reveals a dimer where the functions of dimerization, ATP binding, and regulation are segregated into domains. The kinase domain is unlike Ser/Thr/Tyr kinases but resembles two ATPases, Gyrase B and Hsp90. Structural analogies within this superfamily suggest that the P1 domain of CheA provides the nucleophilic histidine and activating glutamate for phosphotransfer. The regulatory domain, which binds the homologous receptor-coupling protein CheW, topologically resembles two SH3 domains and provides different protein recognition surfaces at each end. The dimerization domain forms a central four-helix bundle about which the kinase and regulatory domains pivot on conserved hinges to modulate transphosphorylation. Different subunit conformations suggest that relative domain motions link receptor response to kinase activity. | ||
==About this Structure== | ==About this Structure== | ||
1B3Q is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Thermotoga_maritima Thermotoga maritima] with HG as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http:// | 1B3Q is a [http://en.wikipedia.org/wiki/Single_protein Single protein] structure of sequence from [http://en.wikipedia.org/wiki/Thermotoga_maritima Thermotoga maritima] with <scene name='pdbligand=HG:'>HG</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1B3Q OCA]. | ||
==Reference== | ==Reference== | ||
| Line 13: | Line 13: | ||
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Thermotoga maritima]] | [[Category: Thermotoga maritima]] | ||
[[Category: Alex, L | [[Category: Alex, L A.]] | ||
[[Category: Bilwes, A | [[Category: Bilwes, A M.]] | ||
[[Category: Crane, B | [[Category: Crane, B R.]] | ||
[[Category: Simon, M | [[Category: Simon, M I.]] | ||
[[Category: HG]] | [[Category: HG]] | ||
[[Category: chemotaxis]] | [[Category: chemotaxis]] | ||
| Line 23: | Line 23: | ||
[[Category: signal transduction]] | [[Category: signal transduction]] | ||
''Page seeded by [http:// | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 11:51:06 2008'' | ||
Revision as of 12:51, 21 February 2008
|
CRYSTAL STRUCTURE OF CHEA-289, A SIGNAL TRANSDUCING HISTIDINE KINASE
OverviewOverview
Histidine kinases allow bacteria, plants, and fungi to sense and respond to their environment. The 2.6 A resolution crystal structure of Thermotoga maritima CheA (290-671) histidine kinase reveals a dimer where the functions of dimerization, ATP binding, and regulation are segregated into domains. The kinase domain is unlike Ser/Thr/Tyr kinases but resembles two ATPases, Gyrase B and Hsp90. Structural analogies within this superfamily suggest that the P1 domain of CheA provides the nucleophilic histidine and activating glutamate for phosphotransfer. The regulatory domain, which binds the homologous receptor-coupling protein CheW, topologically resembles two SH3 domains and provides different protein recognition surfaces at each end. The dimerization domain forms a central four-helix bundle about which the kinase and regulatory domains pivot on conserved hinges to modulate transphosphorylation. Different subunit conformations suggest that relative domain motions link receptor response to kinase activity.
About this StructureAbout this Structure
1B3Q is a Single protein structure of sequence from Thermotoga maritima with as ligand. Full crystallographic information is available from OCA.
ReferenceReference
Structure of CheA, a signal-transducing histidine kinase., Bilwes AM, Alex LA, Crane BR, Simon MI, Cell. 1999 Jan 8;96(1):131-41. PMID:9989504
Page seeded by OCA on Thu Feb 21 11:51:06 2008