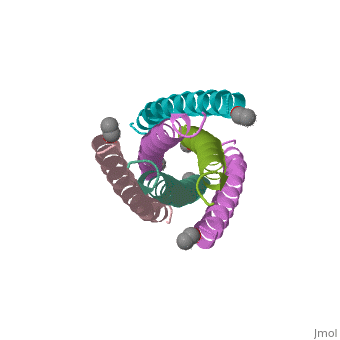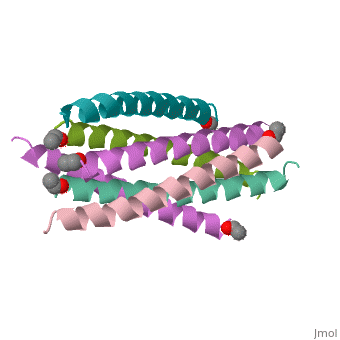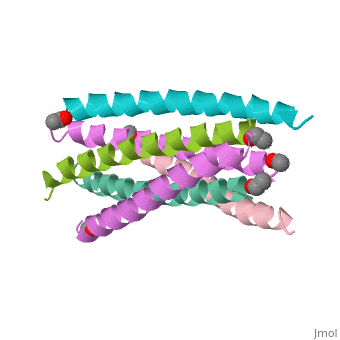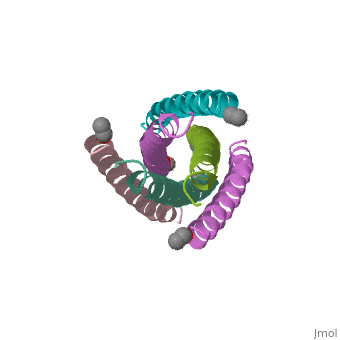1aik: Difference between revisions
New page: left|200px<br /> <applet load="1aik" size="450" color="white" frame="true" align="right" spinBox="true" caption="1aik, resolution 2.0Å" /> '''HIV GP41 CORE STRUCT... |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:1aik.gif|left|200px]]<br /> | [[Image:1aik.gif|left|200px]]<br /><applet load="1aik" size="350" color="white" frame="true" align="right" spinBox="true" | ||
<applet load="1aik" size=" | |||
caption="1aik, resolution 2.0Å" /> | caption="1aik, resolution 2.0Å" /> | ||
'''HIV GP41 CORE STRUCTURE'''<br /> | '''HIV GP41 CORE STRUCTURE'''<br /> | ||
==Overview== | ==Overview== | ||
The envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1) | The envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1) consists of a complex of gp120 and gp41. gp120 determines viral tropism by binding to target-cell receptors, while gp41 mediates fusion between viral and cellular membranes. Previous studies identified an alpha-helical domain within gp41 composed of a trimer of two interacting peptides. The crystal structure of this complex, composed of the peptides N36 and C34, is a six-helical bundle. Three N36 helices form an interior, parallel coiled-coil trimer, while three C34 helices pack in an oblique, antiparallel manner into highly conserved, hydrophobic grooves on the surface of this trimer. This structure shows striking similarity to the low-pH-induced conformation of influenza hemagglutinin and likely represents the core of fusion-active gp41. Avenues for the design/discovery of small-molecule inhibitors of HIV infection are directly suggested by this structure. | ||
==About this Structure== | ==About this Structure== | ||
1AIK is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_hxb2) Human immunodeficiency virus type 1 (isolate hxb2)] with ACE as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http:// | 1AIK is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(isolate_hxb2) Human immunodeficiency virus type 1 (isolate hxb2)] with <scene name='pdbligand=ACE:'>ACE</scene> as [http://en.wikipedia.org/wiki/ligand ligand]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1AIK OCA]. | ||
==Reference== | ==Reference== | ||
| Line 14: | Line 13: | ||
[[Category: Human immunodeficiency virus type 1 (isolate hxb2)]] | [[Category: Human immunodeficiency virus type 1 (isolate hxb2)]] | ||
[[Category: Protein complex]] | [[Category: Protein complex]] | ||
[[Category: Berger, J | [[Category: Berger, J M.]] | ||
[[Category: Chan, D | [[Category: Chan, D C.]] | ||
[[Category: Fass, D.]] | [[Category: Fass, D.]] | ||
[[Category: Kim, P | [[Category: Kim, P S.]] | ||
[[Category: ACE]] | [[Category: ACE]] | ||
[[Category: envelope glycoprotein]] | [[Category: envelope glycoprotein]] | ||
| Line 24: | Line 23: | ||
[[Category: retrovirus]] | [[Category: retrovirus]] | ||
''Page seeded by [http:// | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Thu Feb 21 11:44:52 2008'' | ||
Revision as of 12:44, 21 February 2008
|
HIV GP41 CORE STRUCTURE
OverviewOverview
The envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1) consists of a complex of gp120 and gp41. gp120 determines viral tropism by binding to target-cell receptors, while gp41 mediates fusion between viral and cellular membranes. Previous studies identified an alpha-helical domain within gp41 composed of a trimer of two interacting peptides. The crystal structure of this complex, composed of the peptides N36 and C34, is a six-helical bundle. Three N36 helices form an interior, parallel coiled-coil trimer, while three C34 helices pack in an oblique, antiparallel manner into highly conserved, hydrophobic grooves on the surface of this trimer. This structure shows striking similarity to the low-pH-induced conformation of influenza hemagglutinin and likely represents the core of fusion-active gp41. Avenues for the design/discovery of small-molecule inhibitors of HIV infection are directly suggested by this structure.
About this StructureAbout this Structure
1AIK is a Protein complex structure of sequences from Human immunodeficiency virus type 1 (isolate hxb2) with as ligand. Full crystallographic information is available from OCA.
ReferenceReference
Core structure of gp41 from the HIV envelope glycoprotein., Chan DC, Fass D, Berger JM, Kim PS, Cell. 1997 Apr 18;89(2):263-73. PMID:9108481
Page seeded by OCA on Thu Feb 21 11:44:52 2008