Sandbox 215: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
She also has a fold which is homologous to BPI (a protein which is implicated in lipid binding): two similar domains are connected by a linker. | She also has a fold which is homologous to BPI (a protein which is implicated in lipid binding): two similar domains are connected by a linker. | ||
CETP's structure can be divided into four structural units: | CETP's structure can be divided into four structural units: | ||
At each end of the protein there is a barrel which is constitued of highly twisted B-sheet and two helices called A and B at the N-terminal and A', B' at C-terminal extremity. Helices B and B' are longer than A and A' | * At each end of the protein there is a barrel which is constitued of highly twisted B-sheet and two helices called A and B at the N-terminal and A', B' at C-terminal extremity. Helices B and B' are longer than A and A' | ||
Between the two barrels there is a central B-sheet which is constitued of six antiparallel strands | * Between the two barrels there is a central B-sheet which is constitued of six antiparallel strands | ||
At the C-terminal extremity there is a distorted amphiphathic helix called helix X which is an extension of C-teminal and she interacts with N-terminal residues | * At the C-terminal extremity there is a distorted amphiphathic helix called helix X which is an extension of C-teminal and she interacts with N-terminal residues | ||
Revision as of 16:51, 24 December 2011
| |||||||||
| 2obd, resolution 2.10Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , , , , | ||||||||
| Gene: | CETP (Homo sapiens) | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
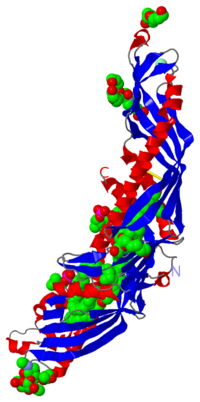
Cholesteryl Ester Transfer Protein is a plasma glycoprotein which is implicated in the transport of cholesteryl esters from the atheroprotective high-density lipoproteins (HDL) to the atherogenic lower-density lipoproteins (LDL). The cristal structure of CETP at 2,2-Å resolution in complex with four bound lipid molecules shows a long tunnel which traverses the core of the molecule and has two distinct large openings allowing lipid access. This tunnel is plugged by an amphiphilic phosphatidylcholine at each end.
Role of CETPRole of CETP
StructureStructure
Overall structureOverall structure
CETP is a 476 amino acid residues protein which has an elongated “boomerang shape” with dimensions of 135A° X 30 A°X 35A°. She has a molecular mass of 74 kDa and 28% of this mass is attributed to N-glycosylation at specific residues: 88, 240, 341 and 396. She also has a fold which is homologous to BPI (a protein which is implicated in lipid binding): two similar domains are connected by a linker. CETP's structure can be divided into four structural units:
- At each end of the protein there is a barrel which is constitued of highly twisted B-sheet and two helices called A and B at the N-terminal and A', B' at C-terminal extremity. Helices B and B' are longer than A and A'
- Between the two barrels there is a central B-sheet which is constitued of six antiparallel strands
- At the C-terminal extremity there is a distorted amphiphathic helix called helix X which is an extension of C-teminal and she interacts with N-terminal residues
