PhoP-PhoQ: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
---- | ---- | ||
'''PhoP-PhoQ''' is a two component regulatory system found in some gram-negative bacteria such as ''Escherichia Coli'', ''Salmonella Typhimurium'', and ''Yersinia Pestis''. In a classic two component regulatory system, there exists a sensor kinase and a response regulator. In the phoP-phoQ system, phoQ acts as the sensor kinase and phoP acts as the response regulator. The purpose of this signal transduction system in bacteria is to modify cellular output in response to environmental signals. In response to particular environmental stimuli, such as a low [Mg<sup>2+</sup>], the sensor kinase, phoQ autophosphorylates. Phosphorylated phoQ then transphosphorylates the response regulator, phoP, which in turn binds DNA and | '''PhoP-PhoQ''' is a two component regulatory system found in some gram-negative bacteria such as ''Escherichia Coli'', ''Salmonella Typhimurium'', and ''Yersinia Pestis''. In a classic two component regulatory system, there exists a sensor kinase and a response regulator. In the phoP-phoQ system, phoQ acts as the sensor kinase and phoP acts as the response regulator. The purpose of this signal transduction system in bacteria is to modify cellular output in response to environmental signals. In response to particular environmental stimuli, such as a low [Mg<sup>2+</sup>], the sensor kinase, phoQ autophosphorylates. Phosphorylated phoQ then transphosphorylates the response regulator, phoP, which in turn binds DNA and modulates transcription. | ||
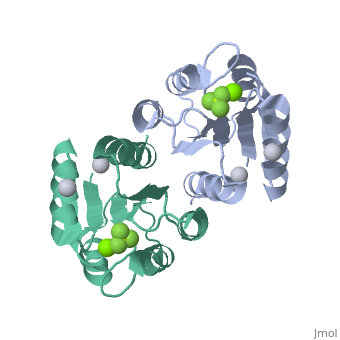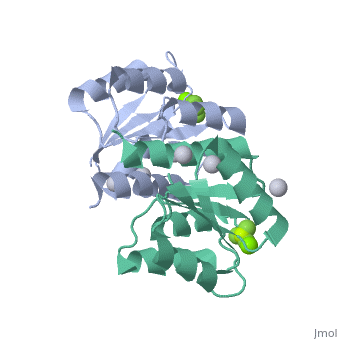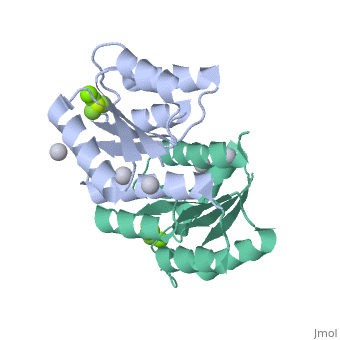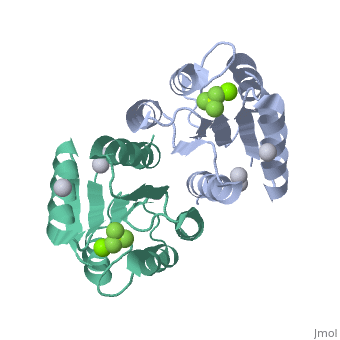
<StructureSection load='2pl1' size='500' side='right' caption='BeF activated PhoP domain of E. Coli (PDB entry [[2pl1]])' scene=''> | <StructureSection load='2pl1' size='500' side='right' caption='BeF activated PhoP domain of E. Coli (PDB entry [[2pl1]])' scene=''> | ||
| Line 16: | Line 16: | ||
E. coli phoP is a 223 residue protein containing a 120-residue regulatory domain joined by a 5-residue linker to a 98 residue c-terminal DNA binding effector domain. At a conserved Asp residue, the regulatory domain can be modified by a phosphoryl group from the protein kinase function of phoQ. Phosphorylation of the regulatory domain modulates the activity of the effector domain to bind DNA and regulate transcription. Phosphorylated, or "activated" phoP binds to "phoP boxes" on bacterial DNA, which consist of two direct hexanucleotide repeats separated by a five nucleotide spacer located at the -35 position: | E. coli phoP is a 223 residue protein containing a 120-residue regulatory domain joined by a 5-residue linker to a 98 residue c-terminal DNA binding effector domain. At a conserved Asp residue, the regulatory domain can be modified by a phosphoryl group from the protein kinase function of phoQ. Phosphorylation of the regulatory domain modulates the activity of the effector domain to bind DNA and regulate transcription. Phosphorylated, or "activated" phoP binds to "phoP boxes" on bacterial DNA, which consist of two direct hexanucleotide repeats separated by a five nucleotide spacer located at the -35 position: | ||
(T/G)GTTTA | |||
===Dimerization of Activated phoP=== | ===Dimerization of Activated phoP=== | ||
| Line 28: | Line 28: | ||
---- | ---- | ||
In this structure, <scene name='Sandbox_Reserved_344/P-analog/1'>Beryllofluoride</scene> (BeF<sub>3</sub><sup>-</sup>) is used as a phosphoryl analog to induce the active state conformation. There are numerous bonds that form as a result of BeF<sub>3</sub><sup>-</sup> coordination. F<sub>1</sub> of BeF<sub>3</sub><sup>-</sup> helps satisfy the octahedral coordination of a present active site <scene name='PhoP-PhoQ/Mg-f1/ | In this structure, <scene name='Sandbox_Reserved_344/P-analog/1'>Beryllofluoride</scene> (BeF<sub>3</sub><sup>-</sup>) is used as a phosphoryl analog to induce the active state conformation. There are numerous bonds that form as a result of BeF<sub>3</sub><sup>-</sup> coordination. F<sub>1</sub> of BeF<sub>3</sub><sup>-</sup> helps satisfy the octahedral coordination of a present active site <scene name='PhoP-PhoQ/Mg-f1/4'>Mg</scene><sup>2+</sup>. The conserved active site lysine, <scene name='PhoP-PhoQ/Lys101-f3/2'>Lys101</scene>, forms important intramolecular and intermolecular salt bridges, one of which is with F<sub>3</sub> of BeF<sub>3</sub><sup>-</sup>. A conserved "switch residue" <scene name='PhoP-PhoQ/Thr79-f2/2'>Thr79</scene>, involved in the activation of all response regulators, forms a H-bond with F<sub>2</sub> of BeF<sub>3</sub><sup>-</sup>. F<sub>2</sub> of BeF<sub>3</sub><sup>-</sup> also forms a H-bond with the backbone nitrogen atom of <scene name='PhoP-PhoQ/Gly53-f2/3'>Gly53</scene>. | ||
</StructureSection> | </StructureSection> | ||
===PhoP-PhoQ and Virulence=== | ===PhoP-PhoQ and Virulence=== | ||
Revision as of 00:57, 16 November 2011
IntroductionIntroduction
PhoP-PhoQ is a two component regulatory system found in some gram-negative bacteria such as Escherichia Coli, Salmonella Typhimurium, and Yersinia Pestis. In a classic two component regulatory system, there exists a sensor kinase and a response regulator. In the phoP-phoQ system, phoQ acts as the sensor kinase and phoP acts as the response regulator. The purpose of this signal transduction system in bacteria is to modify cellular output in response to environmental signals. In response to particular environmental stimuli, such as a low [Mg2+], the sensor kinase, phoQ autophosphorylates. Phosphorylated phoQ then transphosphorylates the response regulator, phoP, which in turn binds DNA and modulates transcription.
PhoP: The Response RegulatorE. coli phoP is a 223 residue protein containing a 120-residue regulatory domain joined by a 5-residue linker to a 98 residue c-terminal DNA binding effector domain. At a conserved Asp residue, the regulatory domain can be modified by a phosphoryl group from the protein kinase function of phoQ. Phosphorylation of the regulatory domain modulates the activity of the effector domain to bind DNA and regulate transcription. Phosphorylated, or "activated" phoP binds to "phoP boxes" on bacterial DNA, which consist of two direct hexanucleotide repeats separated by a five nucleotide spacer located at the -35 position: (T/G)GTTTA Dimerization of Activated phoPThe activated phoP regulatory domain dimerizes with two-fold symmetry using the face formed by . The phosphoryl group donated from phoQ is used to stabilize the active conformation by inducing dimerization. Due to the absence of an intramolecular domain interface which precludes direct transmission of an activation signal, this function as a dimerization motif seems to be the phosphoryl group's only role. Beryllofluoride CoordinationIn this structure, (BeF3-) is used as a phosphoryl analog to induce the active state conformation. There are numerous bonds that form as a result of BeF3- coordination. F1 of BeF3- helps satisfy the octahedral coordination of a present active site 2+. The conserved active site lysine, , forms important intramolecular and intermolecular salt bridges, one of which is with F3 of BeF3-. A conserved "switch residue" , involved in the activation of all response regulators, forms a H-bond with F2 of BeF3-. F2 of BeF3- also forms a H-bond with the backbone nitrogen atom of . |
| ||||||||||
PhoP-PhoQ and VirulencePhoP-PhoQ and Virulence
Most known virulence factors have been isolated by designing laboratory conditions that presumably stimulate environmental signals present in host tissues. However, many pathogenic bacteria do not express their virulence factors until specific host signals are detected, signals which are near impossible to accurately reproduce in a laboratory. To overcome this, a new approach entitled IVET (in vivo expression technology) has been developed. IVET uses animal tissue as the selective medium to enrich bacterial virulence factors specifically induced during infection.
Pathogenic bacteria seldom express virulence genes constitutively, they instead need to be able to express the correct virulence genes in the correct environment. Not all virulence factors confer a selective advantage to the microbe at the same stage of infection. Thus, it is the job of the phoP-phoQ system to modulate virulence gene expression according to the cellular micro-environment.
A particular and well studied environmental factor relative to the phoP-phoQ system is [Mg2+]. In response to low [Mg2+], such as would be found inside a macrophage phagosome, phoQ autophosphorylates and transphorphorylates phoP. PhoP then binds to the bacterial DNA and simultaneously activates the expression of pags (phoP activated genes) and represses the expression of prgs (phoP repressed genes). Among the gene products of pags are proteins necessary to survive inside the macrophage, a critical stage of Salmonella Typhirium virulence. Among the gene products of prgs are proteins necessary for invasion and infection of the host, which are less important once in a host macrophage's phagosome.
3D Structures of PhoP-PhoQ3D Structures of PhoP-PhoQ
PhoPPhoP
3r0j – MtPhoP – Mycobacterium tuberculosis
2pmu – MtPhoP DNA-binding domain
2pkx – EcPhoP regulatory domain (mutant) – Escherichia coli
2pl1 - EcPhoP regulatory domain (mutant) + BeF3
1mvo – PhoP N terminal – Bacillus subtilis
PhoQPhoQ
3cgy – StPhoQ catalytic domain + radicicol – Salmonella typhimurium
3cgz - StPhoQ catalytic domain
1yax - StPhoQ sensor domain (mutant)
3bq8 – EcPhoQ
3bqa – EcPhoQ (mutant)
1id0 – EcPhoQ kinase domain