Plectin: Difference between revisions
Jump to navigation
Jump to search
New page: <Structure load='1sh6' size='500' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /><!-- Please use the "3D" button above this box to inse... |
No edit summary |
||
| Line 5: | Line 5: | ||
--> | --> | ||
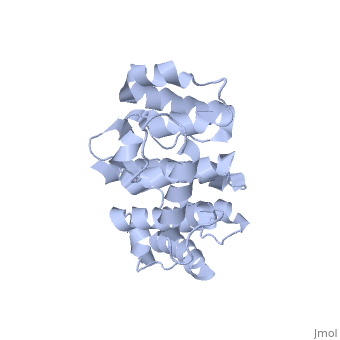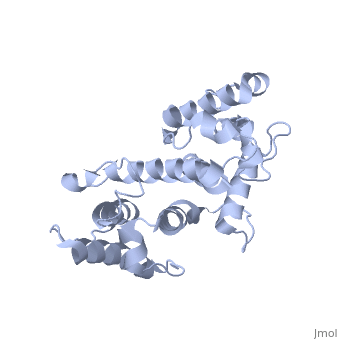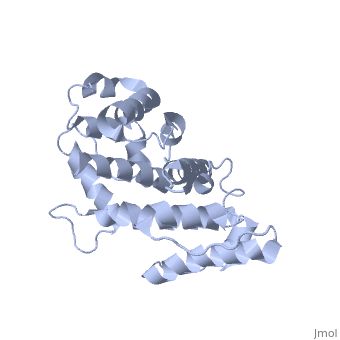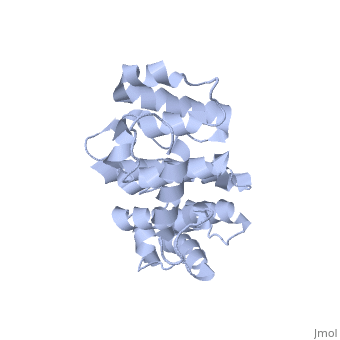
Plectin, a universal and functionally versatile cytolinker protein, can be divided in three main sections; a central coiled-coil rod domain, N and C-terminal globular region and exhibits a dumbbell like structure (1). C-terminal region is composed of 6 homologous repeating domains, and this region has a role in binding to intermediate filaments such as vimentin and cytokeratin (2). N-terminal globular region contains actin binding domain (ABD) comprising two calponin homology (CH) domains and N-terminal arm, which varies among isoforms (3). | Plectin, a universal and functionally versatile cytolinker protein, can be divided in three main sections; a central coiled-coil rod domain, N and C-terminal globular region and exhibits a dumbbell like structure (<ref>PMID:3430617</ref>1). C-terminal region is composed of 6 homologous repeating domains, and this region has a role in binding to intermediate filaments such as vimentin and cytokeratin (2). N-terminal globular region contains actin binding domain (ABD) comprising two calponin homology (CH) domains and N-terminal arm, which varies among isoforms (3). | ||
<references/> | |||
Revision as of 16:16, 23 June 2011
|
Plectin, a universal and functionally versatile cytolinker protein, can be divided in three main sections; a central coiled-coil rod domain, N and C-terminal globular region and exhibits a dumbbell like structure ([1]1). C-terminal region is composed of 6 homologous repeating domains, and this region has a role in binding to intermediate filaments such as vimentin and cytokeratin (2). N-terminal globular region contains actin binding domain (ABD) comprising two calponin homology (CH) domains and N-terminal arm, which varies among isoforms (3).