Fadel A. Samatey Group (Japanese): Difference between revisions
| Line 29: | Line 29: | ||
<!--:<table style="background: #ffd0b0;padding: 6px;"><tr><td>(A few sentences about the main impact could go here ...)</td></tr></table>--> | <!--:<table style="background: #ffd0b0;padding: 6px;"><tr><td>(A few sentences about the main impact could go here ...)</td></tr></table>--> | ||
== | ==OIST以前のサマテの業績== | ||
Before joining OIST, from 1996-2007 Samatey was a member of the Keiichi Namba Group, from 1996 at Matsushita Electric, from 1997 in the [http://www.fbs.osaka-u.ac.jp/labs/namba/npn/index.html ERATO Protonic Nanomachine Project], and from 2002 at [http://www.fbs.osaka-u.ac.jp/eng/labo/09a.html Osaka University], Japan. Samatey earned his Ph.D. in 1992 at [http://www.ujf-grenoble.fr/ Université Joseph Fourier] in Grenoble, France. | Before joining OIST, from 1996-2007 Samatey was a member of the Keiichi Namba Group, from 1996 at Matsushita Electric, from 1997 in the [http://www.fbs.osaka-u.ac.jp/labs/namba/npn/index.html ERATO Protonic Nanomachine Project], and from 2002 at [http://www.fbs.osaka-u.ac.jp/eng/labo/09a.html Osaka University], Japan. Samatey earned his Ph.D. in 1992 at [http://www.ujf-grenoble.fr/ Université Joseph Fourier] in Grenoble, France. | ||
<!-- 1994-6, not yet with Namba [http://pfwww.kek.jp/ Photon Factory] in Tsukuba, Japan --> | <!-- 1994-6, not yet with Namba [http://pfwww.kek.jp/ Photon Factory] in Tsukuba, Japan --> | ||
Revision as of 07:34, 31 May 2011
サマテイ研
Translation of this article to Japanese is in progress.
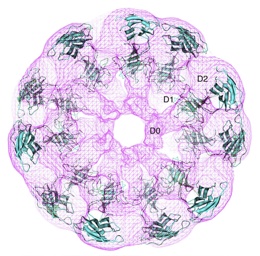 | |
| Crystal structure of flagellar hook fitted into electron density map obtained by electron cryomicroscopy[1]. |
Fadel A. Samatey is Head of the Transmembrane Trafficking Unit at the Okinawa Institute of Science and Technology (OIST) (Japan). Samatey's group uses X-ray crystallography, genetic and biochemical approaches to elucidate the structures and functions of transmembrane proteins, especially type III secretion proteins in bacterial flagella.
Below are listed contributions from the Samatey Group, most recent first.
対象および目的対象および目的
運動性は、生物界において非常に重要な機能である。このために、細菌などの生物は非常に驚くべき分子装置である鞭毛システムを発達させた。大腸菌やネズミチフス菌のような細菌は、この鞭毛と呼ばれる長いらせん状の繊維を回転させて泳ぐことができる。鞭毛は、多くの異なるタンパク質の重合によって作られる複合体で以下の3つの部分に分けることができる。1) 繊維:らせん型のプロペラとして機能する長くて堅い管状構造、2) フック:ユニバーサルジョイントとして働く短くて非常に柔軟な管状部分、および3) 基底小体:細胞膜中に埋め込まれた回転モーター。
鞭毛が形成される過程では、すべての鞭毛軸タンパク質はその中心にある2~3 nmのチャネルを通して細胞質から鞭毛の先端へと輸送される。この輸送メカニズムは、基底小体の細胞質側に位置する特殊なタンパク質輸送系により調節されている。これは、III型輸送装置と呼ばれて細菌界に広く存在している。サルモネラ菌の場合、この輸送装置は6つの膜タンパク質:FlhA、FlhB、FliO、FliP、FliQ、FliRおよび3つの細胞質タンパク質:FliI、FliHおよびFliJから形成されている。また、細菌鞭毛の輸送装置は、グラム陰性病原菌に見られるIII型分泌系(T3SS)に類似している。T3SSの役割は、宿主細胞に対して毒性因子を分泌し、多様な疾患を引き起こすことである。細菌の鞭毛およびT3SSを理解するために、鞭毛タンパク質の構造研究および輸送装置の遺伝学的研究を行っている。
連絡先: f.a.samatey at oist jp
Contributions from OISTContributions from OIST
Samatey joined OIST in 2007.
- ↑ Meshcheryakov VA, Yoon YH, Samatey FA. Purification, crystallization and preliminary X-ray crystallographic analysis of the C-terminal cytoplasmic domain of FlhB from Aquifex aeolicus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Feb 1;67(Pt, 2):280-2. Epub 2011 Jan 27. PMID:21301106 doi:10.1107/S1744309110052942
- ↑ Barker CS, Meshcheryakova IV, Kostyukova AS, Samatey FA. FliO regulation of FliP in the formation of the Salmonella enterica flagellum. PLoS Genet. 2010 Sep 30;6(9). pii: e1001143. PMID:20941389 doi:10.1371/journal.pgen.1001143
OIST以前のサマテの業績OIST以前のサマテの業績
Before joining OIST, from 1996-2007 Samatey was a member of the Keiichi Namba Group, from 1996 at Matsushita Electric, from 1997 in the ERATO Protonic Nanomachine Project, and from 2002 at Osaka University, Japan. Samatey earned his Ph.D. in 1992 at Université Joseph Fourier in Grenoble, France.
 | |
| Simplified model of the rotating bacterial flagellar hook. |
- ↑ Furuta T, Samatey FA, Matsunami H, Imada K, Namba K, Kitao A. Gap compression/extension mechanism of bacterial flagellar hook as the molecular universal joint. J Struct Biol. 2007 Mar;157(3):481-90. Epub 2006 Oct 20. PMID:17142059 doi:10.1016/j.jsb.2006.10.006
Analyses the mechanism by which monomers of the flagellar hook slide against each other during hook rotation, to permit bending while transmitting torque.
- ↑ Kitao A, Yonekura K, Maki-Yonekura S, Samatey FA, Imada K, Namba K, Go N. Switch interactions control energy frustration and multiple flagellar filament structures. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):4894-9. Epub 2006 Mar 20. PMID:16549789 doi:10.1073/pnas.0510285103
Describes a massive molecular dynamics simulation that successfully accounts for polymorphic supercoiling in the bacterial flagellar filament. Interactions between protein monomer chains are dissected into permanent, sliding, and switch categories.
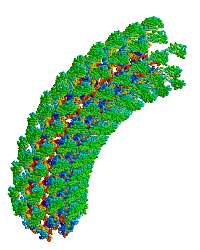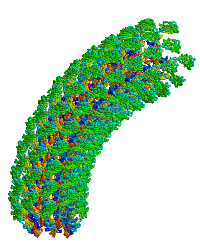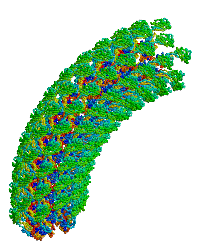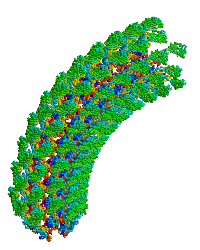 | |
| Bacterial flagellar hook. |
|
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Shaikh TR, Thomas DR, Chen JZ, Derosier DJ, Kitao A, Namba K. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature. 2004 Oct 28;431(7012):1062-8. PMID:15510139 doi:http://dx.doi.org/10.1038/nature02997
Reports the first structure of a major fragment of the protein monomer that assembles into the bacterial flagellar hook (1wlg, 1.8 Å resolution). Fits the monomer into an electron cryomicroscopic density map, resulting in straight and curved models of the hook, including a rotating model.
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Namba K. Crystallization of a core fragment of the flagellar hook protein FlgE. Acta Crystallogr D Biol Crystallogr. 2004 Nov;60(Pt 11):2078-80. Epub 2004, Oct 20. PMID:15502333 doi:10.1107/S0907444904022735
|
- ↑ Samatey FA, Imada K, Nagashima S, Vonderviszt F, Kumasaka T, Yamamoto M, Namba K. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature. 2001 Mar 15;410(6826):331-7. PMID:11268201 doi:10.1038/35066504
Reports, for the first time, the atomic structure of a major fragment of the protein chain monomer that assembles into the bacterial flagellar filament (1io1, 2.0 Å resolution). The crystal contained chains of monomers, which revealed how the monomer protein chains fit together into protofilaments. Theoretical simulation revealed a possible mechanism for how the filament reverses direction, a mechanism crucial to how bacteria swim towards food or away from harm by reversing the flagellar motor.
- ↑ Samatey FA, Imada K, Vonderviszt F, Shirakihara Y, Namba K. Crystallization of the F41 fragment of flagellin and data collection from extremely thin crystals. J Struct Biol. 2000 Nov;132(2):106-11. PMID:11162732 doi:10.1006/jsbi.2000.4312
 | Crystals of the flagellar hook protein produced in the Samatey lab. |
1990's1990's
- ↑ Lazzaroni JC, Vianney A, Popot JL, Benedetti H, Samatey F, Lazdunski C, Portalier R, Geli V. Transmembrane alpha-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995 Feb 10;246(1):1-7. PMID:7853390 doi:http://dx.doi.org/10.1006/jmbi.1994.0058
See AlsoSee Also
ReferencesReferences
- ↑ Samatey FA, Matsunami H, Imada K, Nagashima S, Shaikh TR, Thomas DR, Chen JZ, Derosier DJ, Kitao A, Namba K. Structure of the bacterial flagellar hook and implication for the molecular universal joint mechanism. Nature. 2004 Oct 28;431(7012):1062-8. PMID:15510139 doi:http://dx.doi.org/10.1038/nature02997