Evans sandbox 1: Difference between revisions
No edit summary |
|||
| Line 2: | Line 2: | ||
==General== | ==General== | ||
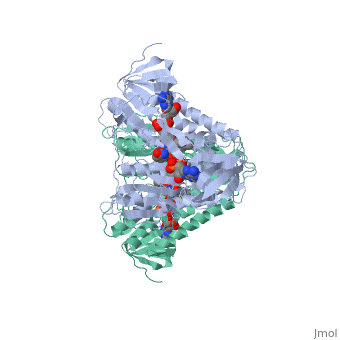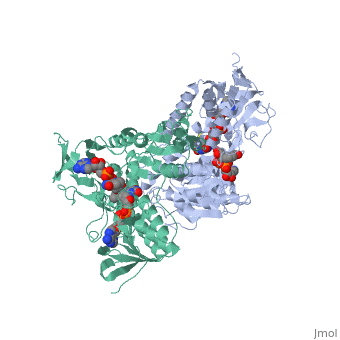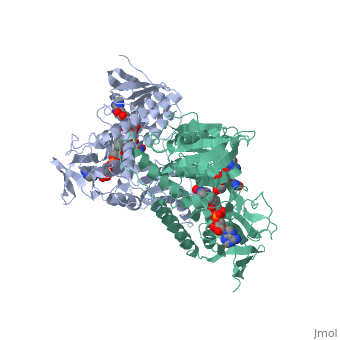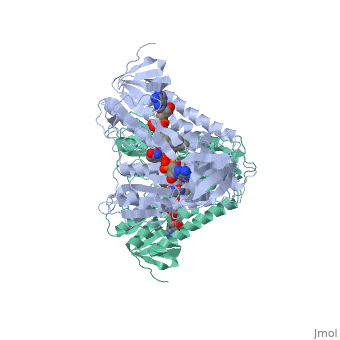
Dihidrolipoamide dehydrogenase (E3), encoded by the DLD gene, belongs to the pyridine nucleotide-disulfide oxidoreductase family including glutathione reductase and thioredoxin reductase. It catalyzes the reoxidation of dihyrolipoyl moiety of the acyltransferase components of three alpha-keto acid deydrogenase complexes and of the hydrogen-carrier protein of the glycine cleavage system. | Dihidrolipoamide dehydrogenase (E3), encoded by the DLD gene, belongs to the pyridine nucleotide-disulfide oxidoreductase family including glutathione reductase and thioredoxin reductase. It catalyzes the reoxidation of dihyrolipoyl moiety of the acyltransferase components of three alpha-keto acid deydrogenase complexes and of the hydrogen-carrier protein of the glycine cleavage system. | ||
==Structure== | ==Structure== | ||
Revision as of 02:28, 27 April 2011
| |||||||||
| 1lvl, resolution 2.45Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Activity: | Dihydrolipoyl dehydrogenase, with EC number 1.8.1.4 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
GeneralGeneral
Dihidrolipoamide dehydrogenase (E3), encoded by the DLD gene, belongs to the pyridine nucleotide-disulfide oxidoreductase family including glutathione reductase and thioredoxin reductase. It catalyzes the reoxidation of dihyrolipoyl moiety of the acyltransferase components of three alpha-keto acid deydrogenase complexes and of the hydrogen-carrier protein of the glycine cleavage system.
StructureStructure
In E. coli this complex exists as 24 E2 proteins arranged in a cube, surrounded by 12 E1 proteins and 12 E3 proteins. Dihidrolipoamide dehydrogenase (E3) binds to the pyruvate dehydrogenase complex (and the center of the cube of E2 proteins) through a .‘[1]’ The E3 binding protein is a completely separate protein from E3, but serves to connect the E3 polypeptides to the overarching structure. The active site includes an FAD group, as well as forming a disulfide bond. When the substrate is not present, “covers” the catalytic site from being exposed to solvents. Dihidrolipoamide dehydrogenase (E3) is a SCOP alpha and beta (a/b) class protein of the FAD/NAD(P)-binding domain fold.
MechanismMechanism
The redox reaction occurs through the influence of , between which there is a disulfide bond within a distorted alpha helix. This redox active disulfide bond becomes reduced in order to reoxidize the E2 enzyme of the multienzyme complex.‘[2]’ E2 donates protons and electrons to E3 in order to complete its catalytic cycle. The E3 enzyme’s flavin ring (FAD) funnels electrons from the disulfide bond to itself, , reoxidizing the E3, and leaving it ready for the beginning of its catalytic cycle again.
RegulationRegulation
The regulation of Dihidrolipoamide dehydrogenase (E3) kinetically comes through regulation of the entire Pyruvate Dehydrogenase complex. As would be expected, one of the main regulators is the presence of its product, acetyl-CoA as well as NADH. This is through the E1 reaction of the complex, but necessarily effects the E3 reaction. However, the E1 portion of the complex is also regulated by phosphatase and kinase in phosphorylation and dephosphorylation reactions.‘[3]’
- ↑ Brautigam CA, Wynn RM, Chuang JL, Machius M, Tomchick DR, Chuang DT. Structural insight into interactions between dihydrolipoamide dehydrogenase (E3) and E3 binding protein of human pyruvate dehydrogenase complex. Structure. 2006 Mar;14(3):611-21. Epub 2006 Jan 26. PMID:16442803 doi:10.1016/j.str.2006.01.001
- ↑ Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. pp.570-575
- ↑ Voet, Donald et al. 2008. Fundamentals of Biochemistry. 3rd ed. p.585