YbgC: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
YbgC is a cytoplasmic protein in the Tol-Pal complex<ref>PMID: 11994151</ref> | {{STRUCTURE_2pzh | PDB=2pzh | SCENE= }} | ||
YbgCis believed to have first appeared in the [[Tol]] complex with the separation of the δε proteobacteria from the αβγ proteobacteria<ref name='Sturgis'>PMID: 11200223</ref>. | |||
==Structure== | |||
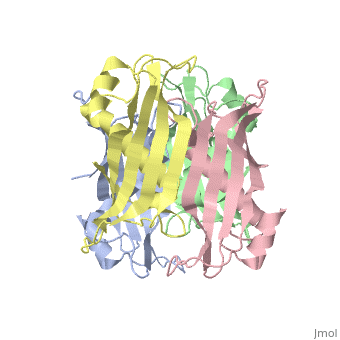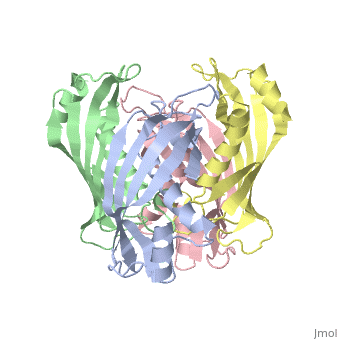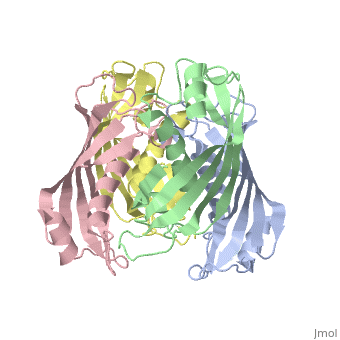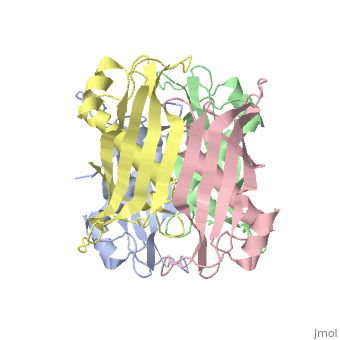
YbgC is a cytoplasmic protein in the Tol-[[Pal]] complex<ref>PMID: 11994151</ref>. In ''Helicobacter pylori'', the protein is part of a 'hot-dog' family of proteins, with an epsilongamma tetrameric arrangement<ref name-'Angelini'>PMID: 18338382</ref>. | |||
==Function== | |||
The protein displays thioesterase activity towards acyl-CoA thioesters<ref name='Angelini'>PMID: 18338382</ref>, and has a strong sequence conservation with its active site residues with other proteins of a similar function<ref>PMID: 9837940</ref> for example with the ''Pseudonomas'' sp. strain CBS3 4-hydroxybenzoyl-CoA thioesterase<ref>PMID: 11959124</ref>. | |||
However, its role within the Tol complex itself remains unknown, although it may be involved in the acetylation of another protein within the complex, with the role of an activity-regulator<ref name='Sturgis'>PMID: 11200223</ref>. It is also thought to be involved in the cell division complex of Tol-Pal<ref name-'Angelini'>PMID: 18338382</ref>. | |||
== References== | == References== | ||
<references/> | <references/> | ||
Revision as of 20:22, 25 April 2011
| |||||||||
| 2pzh, resolution 1.70Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene: | HP0496 (Helicobacter pylori) | ||||||||
| Related: | 1lo7, 1s5u | ||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
YbgCis believed to have first appeared in the Tol complex with the separation of the δε proteobacteria from the αβγ proteobacteria[1].
StructureStructure
YbgC is a cytoplasmic protein in the Tol-Pal complex[2]. In Helicobacter pylori, the protein is part of a 'hot-dog' family of proteins, with an epsilongamma tetrameric arrangement[3].
FunctionFunction
The protein displays thioesterase activity towards acyl-CoA thioesters[4], and has a strong sequence conservation with its active site residues with other proteins of a similar function[5] for example with the Pseudonomas sp. strain CBS3 4-hydroxybenzoyl-CoA thioesterase[6].
However, its role within the Tol complex itself remains unknown, although it may be involved in the acetylation of another protein within the complex, with the role of an activity-regulator[1]. It is also thought to be involved in the cell division complex of Tol-Pal[7].
ReferencesReferences
- ↑ 1.0 1.1 Sturgis JN. Organisation and evolution of the tol-pal gene cluster. J Mol Microbiol Biotechnol. 2001 Jan;3(1):113-22. PMID:11200223
- ↑ Walburger A, Lazdunski C, Corda Y. The Tol/Pal system function requires an interaction between the C-terminal domain of TolA and the N-terminal domain of TolB. Mol Microbiol. 2002 May;44(3):695-708. PMID:11994151
- ↑ Angelini A, Cendron L, Goncalves S, Zanotti G, Terradot L. Structural and enzymatic characterization of HP0496, a YbgC thioesterase from Helicobacter pylori. Proteins. 2008 Mar 12;72(4):1212-1221. PMID:18338382 doi:http://dx.doi.org/10.1002/prot.22014
- ↑ Angelini A, Cendron L, Goncalves S, Zanotti G, Terradot L. Structural and enzymatic characterization of HP0496, a YbgC thioesterase from Helicobacter pylori. Proteins. 2008 Mar 12;72(4):1212-1221. PMID:18338382 doi:http://dx.doi.org/10.1002/prot.22014
- ↑ Benning MM, Wesenberg G, Liu R, Taylor KL, Dunaway-Mariano D, Holden HM. The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. Strain CBS-3. J Biol Chem. 1998 Dec 11;273(50):33572-9. PMID:9837940
- ↑ Zhuang Z, Song F, Martin BM, Dunaway-Mariano D. The YbgC protein encoded by the ybgC gene of the tol-pal gene cluster of Haemophilus influenzae catalyzes acyl-coenzyme A thioester hydrolysis. FEBS Lett. 2002 Apr 10;516(1-3):161-3. PMID:11959124
- ↑ Angelini A, Cendron L, Goncalves S, Zanotti G, Terradot L. Structural and enzymatic characterization of HP0496, a YbgC thioesterase from Helicobacter pylori. Proteins. 2008 Mar 12;72(4):1212-1221. PMID:18338382 doi:http://dx.doi.org/10.1002/prot.22014