Sandbox Reserved 199: Difference between revisions
No edit summary |
|||
| Line 2: | Line 2: | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
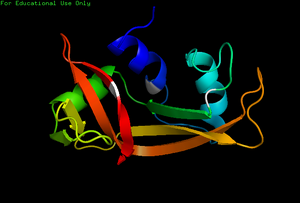
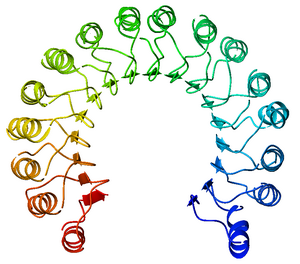
[[Image:Kroupa RNase.png|border | | [[Image:Kroupa RNase.png|border |400px |right |alt=NMR. |2K11-NMR structure of bovine pancreatic RNase.]] | ||
== Introduction == | == Introduction == | ||
Revision as of 05:18, 7 April 2011
| This Sandbox is Reserved from Feb 02, 2011, through Jul 31, 2011 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 191 through Sandbox Reserved 200. |
To get started:
More help: Help:Editing |
IntroductionIntroduction
OverviewOverview
Ribonucleases are some of the most well studied enzymes within the scientific community due to their ample availability as well as their significant role within a cell. In the past, 3D NMR structures of Bovine Pancreatic Ribonuclease (RNase A) and Human Pancreatic Ribonuclease (RNase 1) were obtained. While structures of RNase A and RNase 1 via X-Ray crystallography have been around for some time, the 3D NMR structures present much more information on specific locations of side chain residues as well as their flexibility in the unbound enzymes. Because NMR does not require a "frozen" crystal structure (X-Ray crystallography), NMR imaging can show much more accurate detail into the actual, solution enzyme (folding, flexibility etc.)
Ribonuclease A and Ribonuclease 1 are both good targets for 3D NMR. Not only are they small proteins which make NMR a more feasible option, they also have numerous characteristics that can only be observed in an uncrystallized state, such as internal flexibility and 3D domain swapping.
3D NMR spectroscopy has had shed light on protein folding dynamics as a whole, suggesting a framework model of folding (Folding order = primary structure, secondary structure, tertiary structure).
NMR Versus X-Ray CrystallographyNMR Versus X-Ray Crystallography
The two predominant methods of protein tertiary structure determination are X-Ray Crystallographyand Nuclear Magnetic Resonance(NMR) Spectroscopy.
X-Ray Crystallography entails protein purification, crystallization of the protein, collection of X-Ray diffraction data, calculation of the protein’s electron density relative to the determined data, and finally fitting the protein’s determined residue sequence into the electron density. Crystallization of the protein often lends itself to being the most challenging aspect of this method. While any size protein can be studied via X-Ray Crystallography and the method is well-established, it is often difficult to perform for membrane proteins and the data received reveals no information about the protein’s hydrogen atoms. Also, an assumption made for X-Ray Crystallographic studies is that the crystallized protein is in a conformation similar to that seen in solution. For more information regarding X-Ray Crystallography please click here.
Bimolecular NMR Spectroscopy involves protein purification, dissolving the protein in a suitable solvent, collecting the NMR data, assigning NMR signals, and finally calculating the protein’s tertiary structure. With NMR, the most difficult step is often correctly assigning NMR signals. Although there is no need to crystallize the protein of interest and most hydrogen atoms are evident, NMR is difficult for proteins that don’t dissolve well in common solvents and works best for small proteins. 1-dimensional, 2-dimensional, and 3-dimensional NMR spectroscopy is readily available; however, 2-dimensional and 3-dimensional NMR is most often used for protein tertiary structure determination. 2D NMR reveals chemical shift correlations between spinnable nuclei such as 1H, 13C, 15N, and 13P, as well as atomic coupling, or proximity correlations via bonding. 3D NMR utilizes this methodology in addition to detection of another nuclear spin phenomenon known as the Nuclear Overhauser Effect(NOE), in which proximity correlations are can be observed in 3D space.
Due to the complexity of assigning NMR signals to specific protons, the data gathered is usually put into a computer that uses complex computer algorithms to render a protein’s tertiary structure. Even though NMR often requires more prior knowledge of the studied protein’s structural information, the end result is often more telling than that of X-Ray Crystallography. With NMR, one can gain a better insight as to what exactly a folded protein might look like in solution including the protein’s conformational flexibility. An example is the many-conformational structure of bovine pancreatic Ribonuclease seen when the page originally loads. For more information regarding NMR Spectroscopy, click here.
History of NMR Ribonuclease StudiesHistory of NMR Ribonuclease Studies
In 1957, the first work was published examining the structure of bovine pancreatic Ribonuclease using 1-Dimensional 1H NMR by Martin Saunder et al.
In 1988, Udgaonkar et al. used 2-dimensional 1H NMR to study bovine pancreatic Ribonuclease and examined protein folding dynamics, which supported the framework model protein folding mechanism.
In 1993, Santoro et al. used 3-dimensional 1H NMR to study bovine pancreatic Ribonuclease to compare NMR structures with X-Ray Crystallography structures.
In 2008, Rico et al. used 3-dimensional 1H NMR to study human pancreatic Ribonuclease. They looked at active site conformational changes upon substrate binding, and suggested possible biological implications of their findings.
Medical SignificanceMedical Significance
Ribonucleases which can prevent inhibition by ribonuclease inhibitors have been looked at as potential anti-tumor agents for some time now. In fact, Onconase ® (an RNase A homolog from the green leopard frog) is already in clinical trials due to its toxicity to cancer cells. However, due to possible immunogenicity of the frog enzyme, much effort has been focused on developing a mutated human RNase which can prevent inhibition by human ribonuclease inhibitor (HcRI). HcRI selectively binds to human pancreatic RNase (RNase 1) and impedes its enzymatic activity. Without sufficient degradation of mRNA, the cell undergoes enhanced gene expression and can lead to cancer. Correctly characterizing RNase 1’s structure and binding specificity via NMR will prove vital to the development of RNase-based anti-cancer treatments.
NMR Study of Ribonuclease Folding Dynamics[1]NMR Study of Ribonuclease Folding Dynamics[1]
Experimental ProcedureExperimental Procedure
Using 2-dimensional 1H NMR, Udgaonkar et al. studied the folding pathway of bovine pancreatic Ribonuclease using an exchange reaction between with solvent protons. 2- dimensional 1H NMR allowed for monitoring of proton exchange in the amide backbone for ten second time intervals, and this proton labeling could be terminated via a rapid drop in pH reaction conditions. This research focused on initial protein folding steps.
Starting with denatured wt Ribonuclease, it was suggested that as the peptide began to fold, the backbone amide proteins would become less energetically favorable to exchange protons with the solvent as the backbone amide protons became involved in folding-related intermolecular interactions (such as ).
Data and ResultsData and Results
became evident as those involved in folding-related intermolecular interactions during initial protein folding steps: Those associated with , , , , and . All five of these protons are involved in hydrogen bonding within the of Ribonuclease; therefore, it was believed that this secondary structure was the starting point for the folding mechanism of Ribonuclease. Furthermore, this suggests the formation of a stable secondary structure before the formation of the , which is consistent with the framework model of protein folding mechanisms (in comparison with the jigsaw puzzle model).
|
Ribonuclease NMR Structure Versus X-Ray Crystallography Ribonuclease Structure[2]Ribonuclease NMR Structure Versus X-Ray Crystallography Ribonuclease Structure[2]
Experimental ProcedureExperimental Procedure
Using 3-dimensional 1H NMR (2D NMR with NOE enhancement), Santoro et al. studied the tertiary structure of bovine pancreatic Ribonuclease. Their experimental raw data were processed by TRITON software, and previously determined proton assignments were utilized. The NMR tertiary structure results were categorized by their quality, or by the size and number of residual distance constraint violations. Using an equation from X-Ray Crystallography studies, the reliability factor (R-Factor) of each structure can be defined by comparing experimental NOE intensities with theoretical NOE intensities. The R-Factor can be defined as:
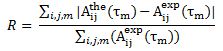
Where A(the) is the theoretical NOE intensity and A(exp) is the experimentally determined NOE value.
Data and ResultsData and Results
The NMR experiment yielded an overall R-Factor of 0.44, compared to an X-Ray Crystallographic R-Factor of 0.45. This means that the NMR structure shows a higher structural reliability compared to the X-Ray Crystallographic structure. Overall, the bovine Ribonuclease NMR tertiary structure matches closely the corresponding X-Ray Crystallography structure. The overall shape, main-chain fold, and side chain positions of most residues are similar between the structures of the two methods. Experimentally determined tertiary structural differences between the two methods were suggested to be due to pH differences, crystal packing, solvation, and temperature variability.
Previously, researchers found the side chain position of in the enzyme’s () of NMR structures to be different than that of X-Ray Crystallography studies. Crystals show a static position of this His 119 residue, yet NMR structures suggest a dynamic equilibrium between the two conformational puckers of the . This single residue difference between crystal and solution studies amplifies to cause a major difference in surrounding amino acid residues: , , and . The researchers proposed that this difference is most likely due to pH induced charge repulsion of His 119 with and in solution.
More than 60 main-chain hydrogen bonds were observed, which closely corresponds to the number of hydrogen bonds determined in crystals; however, there exist a few discrepancies. In the NMR structure, there was determined to be a hydrogen bond between the amide proton on , as well as between . The researchers suggested these differences are most likely due to the same pH phenomenon mentioned above. Other hydrogen bonds present in the NMR structure but not present in the crystal structure are: , , and .
The researchers also utilized the NMRs advantage of determining flexibility of Ribonuclease. Overall, the largest conformational flexibility resulted within the side-chains. Specifically, side-chain mobility is greatest in residues (shown in white). As expected, the backbone torsion angles were seen to be more rigid (less conformational flexibility) within the of Ribonuclease.
Solution Structure and Dynamics of Human Pancreatic Ribonuclease[3]Solution Structure and Dynamics of Human Pancreatic Ribonuclease[3]
|
Experimental ProcedureExperimental Procedure
This study builds on the results of the previously mentioned experiment. Using 3D NMR, Rico et al. obtained the first NMR 3-dimensional structures of Human Rnase 1. Using 20 different RNase NMR structures, researchers compared the RMSD values of key RNase 1 residues. A residue with a high RMSD value has more flexibility and a residue’s ability to adapt to numerous conformations may be crucial to its active role within the enzyme.
Furthermore, this study characterized the dimerizational prperties of wt Ribonuclease and mutated Ribonuclease variants. Similar dimerization, observed in certain Bovine RNases, has shown to greatly enhance enzymatic activity and have augmented anti-tumoral action. Using varying enzyme concentrations and pH conditions as well as site-directed mutations, researchers were able to promote dimerization of RNase 1 variants.
This study also compares the location of certain residues in HcrI bound RNase 1 to unbound RNase. Through this comparison, these researchers provide greater insight into the catalytic mechanism and substrate specificity of RNase 1.
Data and ResultsData and Results
A 3D NMR structure was obtained with a backbone RMSD of 1.07Å. The obtained model shows a similar tertiary structure to the kidney bean shaped RNase A and is stabilized by four . The structure shows three and seven . While this structure matches up fairly well with previous X-Ray crystallography structures of RNase 1, important differences in residue positioning can be seen in the which are not apparent in X-Ray crystallography. Specifically, certain residues with more flexibility undergo a significant conformational change when bound to certain substrates, such as the human ribonuclease inhibitor (HcrI). These residues include: .
This data suggests an “induced-fit” model of substrate binding and may prove vital to fully understanding RNase 1’s binding specificity for Hcrl; although two residues, , show much more rigidity and possibly contribute some “lock-and-key” binding interaction.
15N NMR relaxation shows increased T1 values for the residues found in these sheets and loops (0.63-0.64s relative to 0.60s in helices). This suggests greater flexibility in these regions as well.
Interestingly, the global correlation time of RNase 1 was shown to be much longer than what is expected for a 13.7 kDa monomer (10ns compared to 6-7ns). This shows that under NMR sample conditions, the RNase1 undergoes dimerization forming a monomer/dimer equilibrium. Because dimerization has shown to have a significant effect on enzyme activity in bovine RNases, certain mutated RNase 1’s which show increased dimerization may be potential anti-cancer therapeutics.
ReferencesReferences
- ↑ Saunders, Martin, Arnold Wishnia, and John G. Kirkwood. "The Nuclear Magnetic Resonance Spectrum of Ribonuclease." Communications to the Editor 79; 20 May (1957). Print.
- ↑ Udgaonkar, Jayant B., and Robert L. Baldwin. "NMR evidence for an early framework intermediate on the folding pathway of ribonuclease A." Nature 335.1; 20 Oct. (1988). Print.
- ↑ Santoro, Jorge. "High-resolution Three-dimensional Structure of Ribonuclease A in Solution by Nuclear Magnetic Resonance Spectroscopy." Journal of Molecular Biology 229 (1993). Print.
- ↑ Rico, M. "The Solution Structure and Dynamics of Human Pancreatic Ribonuclease Determined by NMR Spectroscopy Provide Insight into Its Remarkable Biological Activities and Inhibition." Journal of Molecular Biology 379; 14 Apr. (2008). Print.
External ResourcesExternal Resources
Daniel Kroupa 05:03, 30 March 2011 (IST)