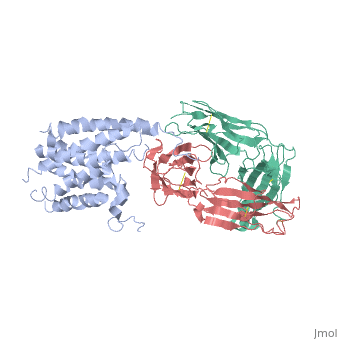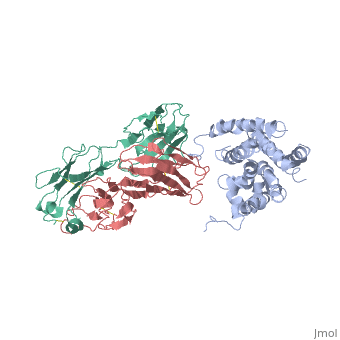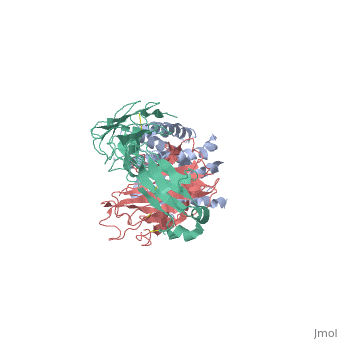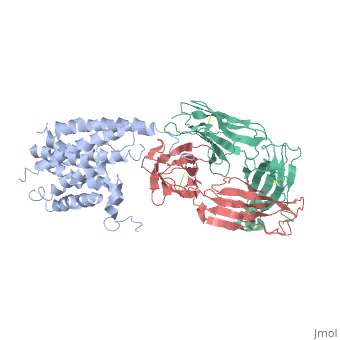2r4r: Difference between revisions
New page: left|200px<br /> <applet load="2r4r" size="450" color="white" frame="true" align="right" spinBox="true" caption="2r4r, resolution 3.400Å" /> '''Crystal structure ... |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Image:2r4r. | [[Image:2r4r.jpg|left|200px]]<br /><applet load="2r4r" size="350" color="white" frame="true" align="right" spinBox="true" | ||
<applet load="2r4r" size=" | |||
caption="2r4r, resolution 3.400Å" /> | caption="2r4r, resolution 3.400Å" /> | ||
'''Crystal structure of the human beta2 adrenoceptor'''<br /> | '''Crystal structure of the human beta2 adrenoceptor'''<br /> | ||
| Line 6: | Line 5: | ||
==Overview== | ==Overview== | ||
Structural analysis of G-protein-coupled receptors (GPCRs) for hormones, and neurotransmitters has been hindered by their low natural abundance, inherent structural flexibility, and instability in detergent solutions., Here we report a structure of the human beta(2) adrenoceptor (beta(2)AR), which was crystallized in a lipid environment when bound to an inverse, agonist and in complex with a Fab that binds to the third intracellular, loop. Diffraction data were obtained by high-brilliance, microcrystallography and the structure determined at 3.4 A/3.7 A, resolution. The cytoplasmic ends of the beta(2)AR transmembrane segments, and the connecting loops are well resolved, whereas the extracellular, regions of the beta(2)AR are not seen. The beta(2)AR structure differs, from rhodopsin in having weaker interactions between the cytoplasmic ends, of transmembrane (TM)3 and TM6, involving the conserved E/DRY sequences., These differences may be responsible for the relatively high basal, activity and structural instability of the beta(2)AR, and contribute to, the challenges in obtaining diffraction-quality crystals of non-rhodopsin, GPCRs. | Structural analysis of G-protein-coupled receptors (GPCRs) for hormones, and neurotransmitters has been hindered by their low natural abundance, inherent structural flexibility, and instability in detergent solutions., Here we report a structure of the human beta(2) adrenoceptor (beta(2)AR), which was crystallized in a lipid environment when bound to an inverse, agonist and in complex with a Fab that binds to the third intracellular, loop. Diffraction data were obtained by high-brilliance, microcrystallography and the structure determined at 3.4 A/3.7 A, resolution. The cytoplasmic ends of the beta(2)AR transmembrane segments, and the connecting loops are well resolved, whereas the extracellular, regions of the beta(2)AR are not seen. The beta(2)AR structure differs, from rhodopsin in having weaker interactions between the cytoplasmic ends, of transmembrane (TM)3 and TM6, involving the conserved E/DRY sequences., These differences may be responsible for the relatively high basal, activity and structural instability of the beta(2)AR, and contribute to, the challenges in obtaining diffraction-quality crystals of non-rhodopsin, GPCRs. | ||
==About this Structure== | ==About this Structure== | ||
2R4R is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus]. Full crystallographic information is available from [http:// | 2R4R is a [http://en.wikipedia.org/wiki/Protein_complex Protein complex] structure of sequences from [http://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2R4R OCA]. | ||
==Reference== | ==Reference== | ||
| Line 42: | Line 38: | ||
[[Category: transmembrane helix]] | [[Category: transmembrane helix]] | ||
''Page seeded by [http:// | ''Page seeded by [http://oca.weizmann.ac.il/oca OCA ] on Wed Jan 23 12:24:06 2008'' | ||
Revision as of 13:24, 23 January 2008
|
Crystal structure of the human beta2 adrenoceptor
OverviewOverview
Structural analysis of G-protein-coupled receptors (GPCRs) for hormones, and neurotransmitters has been hindered by their low natural abundance, inherent structural flexibility, and instability in detergent solutions., Here we report a structure of the human beta(2) adrenoceptor (beta(2)AR), which was crystallized in a lipid environment when bound to an inverse, agonist and in complex with a Fab that binds to the third intracellular, loop. Diffraction data were obtained by high-brilliance, microcrystallography and the structure determined at 3.4 A/3.7 A, resolution. The cytoplasmic ends of the beta(2)AR transmembrane segments, and the connecting loops are well resolved, whereas the extracellular, regions of the beta(2)AR are not seen. The beta(2)AR structure differs, from rhodopsin in having weaker interactions between the cytoplasmic ends, of transmembrane (TM)3 and TM6, involving the conserved E/DRY sequences., These differences may be responsible for the relatively high basal, activity and structural instability of the beta(2)AR, and contribute to, the challenges in obtaining diffraction-quality crystals of non-rhodopsin, GPCRs.
About this StructureAbout this Structure
2R4R is a Protein complex structure of sequences from Homo sapiens and Mus musculus. Full crystallographic information is available from OCA.
ReferenceReference
Crystal structure of the human beta(2) adrenergic G-protein-coupled receptor., Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK, Nature. 2007 Oct 21;. PMID:17952055
Page seeded by OCA on Wed Jan 23 12:24:06 2008
Proteopedia Page Contributors and Editors (what is this?)Proteopedia Page Contributors and Editors (what is this?)
OCA- Pages with broken file links
- Homo sapiens
- Mus musculus
- Protein complex
- Burghammer, M.
- Choi, H.J.
- Edwards, P.C.
- Fischetti, R.F.
- Kobilka, B.K.
- Kobilka, T.S.
- Rasmussen, S.G.
- Ratnala, V.R.
- Rosenbaum, D.M.
- Sanishvili, R.
- Schertler, G.F.
- Thian, F.S.
- Weis, W.I.
- G-protein coupled receptor
- Glycoprotein
- Lipoprotein
- Palmitate
- Phosphorylation
- Polymorphism
- Receptor
- Signaling protein
- Transducer
- Transmembrane helix