Cocaethylene Synthesis and Pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 44: | Line 44: | ||
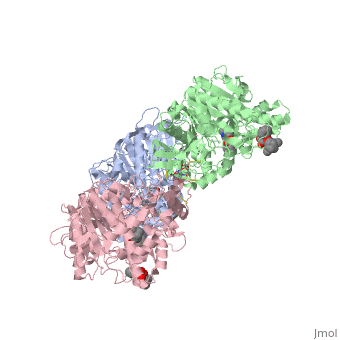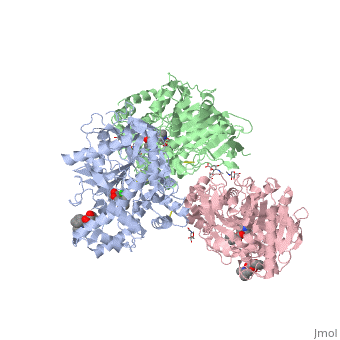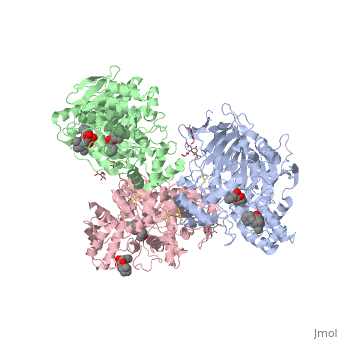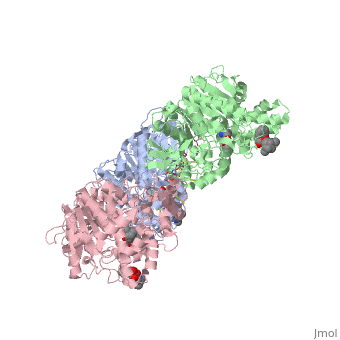
In 2003, a crystal structure of hCE1 was developed using X-ray diffraction techniques to obtain a resolution of 2.80 Angrstoms. The structure was shown in complex with <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Homatropine/1'>homatropine</scene>, a cocaine analogue, and it comprised of two trimers for a total of six identical subunits. Each subunit is 548 amino acids long and each is equipped with two catalytic binding sites to process cocaine. Each trimer subunit associates with a <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Chlorine/3'>chlorine ion</scene>, and the overall protein is aptly named a glycoprotein due to ligand interactions forming with <scene name='Cocaethylene_Synthesis_and_Pathophysiology/N-acetyl-d-glucosamine/1'>n-acetyl-d-glucosamine</scene>, <scene name='Cocaethylene_Synthesis_and_Pathophysiology/2-acetylamino-2-deoxy-a-d-gl/1'>2-(acetylamino)-2-deoxy-a-d-glucopyranose</scene>, and <scene name='Cocaethylene_Synthesis_and_Pathophysiology/O-sialic_acid/1'>o-sialic acid.</scene> | In 2003, a crystal structure of hCE1 was developed using X-ray diffraction techniques to obtain a resolution of 2.80 Angrstoms. The structure was shown in complex with <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Homatropine/1'>homatropine</scene>, a cocaine analogue, and it comprised of two trimers for a total of six identical subunits. Each subunit is 548 amino acids long and each is equipped with two catalytic binding sites to process cocaine. Each trimer subunit associates with a <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Chlorine/3'>chlorine ion</scene>, and the overall protein is aptly named a glycoprotein due to ligand interactions forming with <scene name='Cocaethylene_Synthesis_and_Pathophysiology/N-acetyl-d-glucosamine/1'>n-acetyl-d-glucosamine</scene>, <scene name='Cocaethylene_Synthesis_and_Pathophysiology/2-acetylamino-2-deoxy-a-d-gl/1'>2-(acetylamino)-2-deoxy-a-d-glucopyranose</scene>, and <scene name='Cocaethylene_Synthesis_and_Pathophysiology/O-sialic_acid/1'>o-sialic acid.</scene> | ||
While there are two binding sites for homatropine on each monomer, only one is catalytically active. The <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Activesite/ | While there are two binding sites for homatropine on each monomer, only one is catalytically active. The homatropine molecule must first enter the catalytic site through the <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Bindinggorge/1'>binding gorge</scene>. Once the homatropine (blue) molecule has entered through the pocket, it can then bind to the <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Activesite/8'>active site</scene> of hCE1 which is characterized by a classic catalytic triad comprised of the following residues: Serine221 (orange), Glutamate354 (yellow), and Histidine468 (black). Directly adjacent to the homatropine molecule in the active site is an <scene name='Cocaethylene_Synthesis_and_Pathophysiology/Oxyanion_red_pocket/1'>oxyanion pocket (red)</scene> which is comprised of the two amide nitrogens of two adjacent glycine residues. These nitrogens serve to stabilize the polar tetrahedral acyl-enzyme intermediate formed in an otherwise relatively hydrophobic enzyme pocket. It should be noted that the presence of the S-enantiomer of cocaine in this pocket forms a steric clash with the oxyanion pocket and thus prevents it from being hydrolyzed. | ||
Revision as of 12:41, 30 November 2010

Case StudyCase Study
A 24-year-old Caucasian male patient presents with a stabbing pain behind the eyes, sharp and piercing chest pain, inability to stand or elevate the arms, and loss of vision in the right eye. Patient has a history of hypertension and doctors have noted the presence of Marfan’s traits. Marfan’s syndrome is a disease caused by a mutated fibrillin protein which is involved in the integrity of the extracellular matrix for formation of elastic fibers which are abundant in the blood vessel walls and various other tissues in the body. Patient also has a history of drug and alcohol abuse since the third grade. Abuse of cocaine began in high school and the patient reports to co-administration of alcohol and cocaine on at least fifteen separate occasions with ten of those incidents occurring in the last two years.
BackgroundBackground
Cocaine is a prevalent drug in America with over 15% of the general population (above age 12) admitting usage at least once in their lifetime. Approximately 30% of all drug-related emergency department visits in 2006 were related to cocaine and it has been reported that anywhere from 30-60% of people who abuse cocaine also consume alcohol concomitantly. In fact, a 1989 study put forth by the Drug Abuse Warning Network (DAWN) showed that 75% of all cocaine related deaths in America occurred in patients that were also under the influence of additional drugs, the majority of which were ethanol.
The significant occurrence of fatalities in patients that ingest alcohol and cocaine concurrently is supported by a growing body of evidence that shows increases in sudden death by as much as 25-fold as well as experiments on lab animals that show a lower lethal dosage to kill 50% of subjects (LD50) when this combination is administered.
Pathophysiology of AlcoholPathophysiology of Alcohol
Alcohol alone promotes a drop in blood pressure by at least two mechanisms:
First, alcohol promotes the vasodilation of peripheral blood vessels which is the primary cause of the warm flushing feeling a person experiences when they have ingested ethanol. A dilation of blood vessels causes an increase in blood delivery whilst decreasing the resistance the blood has to encounter when being pumped across a vessel wall. This decrease in resistance essentially lowers the force by which the heart has to pump and thus decreases the blood pressure.
Second, alcohol also inhibits the secretion of vasopressin from the anterior pituitary gland. Vasopressin is responsible for preventing water loss by promoting re-absorption in the collecting duct of the nephron in the kidneys. Thus, alcohol’s inhibition of vasopressin causes a loss of water, which in turn causes a drop in blood pressure because the heart has to do less work to move less volume in the cardiovascular system. However, the oxygen demands of the body remain the same and the body has to compensate with a reflex mechanism that elevates the heart rate and maintains adequate blood flow to all the tissues.
Alcohol is metabolized first by alcohol dehydrogenase into the relatively carcinogenic compound acetaldehyde. Acetaldehyde is then converted enzymatically into acetic acid which is then converted into acetyl-CoA which can enter the citric acid cycle.
Pathophysiology of CocainePathophysiology of Cocaine
Cocaine, on the other hand, can be metabolized by any one of three esterase enzymes into water soluble metabolites. Intestinal carboxylesterase and serum butyrylcholinesterase hydrolyze the benzoyl ester linkage of cocaine, but these two metabolic pathways are relatively insignificant. The chief degradation of cocaine occurs via the catalytic properties of human carboxylesterase 1 (hCE1) which hydrolyzes the methyl ester linkage on cocaine to form benzoylecgonine and methanol.
The primary physiological effects of cocaine ingestion are manifested by direct stimulation of the sympathetic nervous system in humans. The chief neuro-transmitters that mediate the properties of this stimulant are nor-epinephrine and dopamine. All three of these catecholamine neurotransmitters are released in some small level in a normal human being to maintain homeostatic parameters (i.e. blood pressure, temperature, and heart rate). However, these transmitters are released at neural synapses and reabsorbed constantly through special channels in the axon terminal. Cocaine acts to bind these reuptake channels and inhibits the return of these catecholamines which results in a build up at the synaptic cleft causing excessive stimulation of the postsynaptic catecholamine receptors. The cardiac postsynaptic receptors that respond to the buildup of catecholamines are the β1-adrenoceptors which become over-stimulated when cocaine is ingested. Activation of the β1-adrenoceptors causes a number of cascades which leads to an increase force of contraction and heart rate, namely:

1.) β1-adrenoceptors increase intracellular cAMP which activates protein kinases to phosphorylate calcium channels which increases inward calcium which increases contraction by the calcium-dependent troponin contractile complex in cardiac muscle
2.) β1-adrenoceptors also increases the internal automaticity of the heart which, among other factors, owes to the elevation of the heart rate by decreasing the slope of the cardiac action potential in the pacemaker
Ethanol + Cocaine PathophysiologyEthanol + Cocaine Pathophysiology
When alcohol and cocaine are ingested simultaneously, a synergistic effect has been documented which have been attributed to the properties of the enzyme that is chiefly responsible for cocaine degradation (namely hCE1). In the presence of both ethanol and cocaine, it has been well established that a third metabolite, cocaethylene, is produced via hCE1 catalysis of the trans-esterification of the methyl group on cocaine with the ethyl group in ethanol. While the precise mechanisms and interactions which cause greater toxicity in alcohol-cocaine combinations is not crystal clear, there are several experimental observations that account for significant increase in death when this combination is administered, namely:
- 1.) Cocaethylene acts very similar to cocaine in inhibiting reuptake of dopamine and norepinephrine at the neural synapse—it should be noted that cocaethylene is an even more potent reuptake inhibitor of dopamine (leading to more pronounced feelings of pleasure)
- 2.) The half-life of cocaine is only 38 minutes, while the half-life of cocaethylene is 2.5 hours thus resulting in a prolonged period of euphoria before symptoms of dysphoria or “crash” present
- 3.) It has been suggested that binding of serotonin to cocaine results in the onset of dysphoria because cocaine can no longer inhibit reuptake—cocaethylene on the other hand has a 40-fold reduced affinity for serotonin and thus is less restricted from inhibiting catecholamine reuptake channels
- 4.) Ethanol molecules, which occupy a small side-door pocket in hCE1, has been implicated as an inhibitor of cocaine hydrolysis which would account for a reported 30% elevation of plasma cocaine concentrations in the presence of ethano
- 5.) Cocaethylene, itself being a product of the trans-esterification reaction, acts as a competitive inhibitor for the substrate binding pocket on hCE1 which causes a reduction in the hydrolysis of cocaine
Human Carboxylesterase 1Human Carboxylesterase 1
| |||||||||
| 1mx5, resolution 2.80Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , | ||||||||
| Activity: | Carboxylesterase, with EC number 3.1.1.1 | ||||||||
| Related: | 1mx1, 1mx9 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
hCE1 is a promiscuous drug metabolizing enzyme which is encoded by the CES1 gene and is expressed in the liver, small intestine, kidney, lung, testes, heart, monocytes, macrophages, and blood. hCE1 promiscuity allows for processing of such compounds as lovastatin, lidocaine, heroin, a number of military grade chemical gases, and cocaine. In the human body, hCE1 converts cocaine into benzyolecgonine and methanol by hydrolyzing the methyl ester linkage. In the presence of alcohol, however, hCE1 catalyzes a transesterification of the methyl and ethyl ester linkages to form cocaethylene.
In 2003, a crystal structure of hCE1 was developed using X-ray diffraction techniques to obtain a resolution of 2.80 Angrstoms. The structure was shown in complex with , a cocaine analogue, and it comprised of two trimers for a total of six identical subunits. Each subunit is 548 amino acids long and each is equipped with two catalytic binding sites to process cocaine. Each trimer subunit associates with a , and the overall protein is aptly named a glycoprotein due to ligand interactions forming with , , and
While there are two binding sites for homatropine on each monomer, only one is catalytically active. The homatropine molecule must first enter the catalytic site through the . Once the homatropine (blue) molecule has entered through the pocket, it can then bind to the of hCE1 which is characterized by a classic catalytic triad comprised of the following residues: Serine221 (orange), Glutamate354 (yellow), and Histidine468 (black). Directly adjacent to the homatropine molecule in the active site is an which is comprised of the two amide nitrogens of two adjacent glycine residues. These nitrogens serve to stabilize the polar tetrahedral acyl-enzyme intermediate formed in an otherwise relatively hydrophobic enzyme pocket. It should be noted that the presence of the S-enantiomer of cocaine in this pocket forms a steric clash with the oxyanion pocket and thus prevents it from being hydrolyzed.