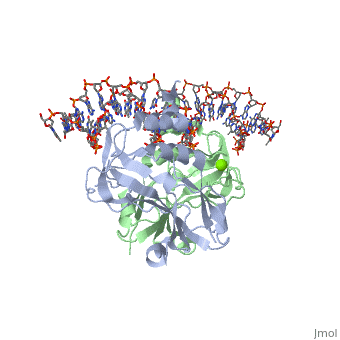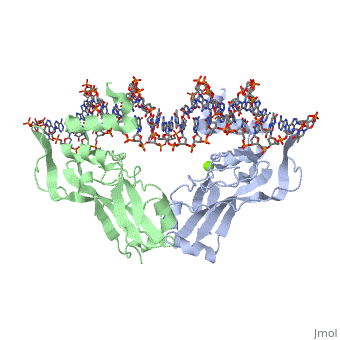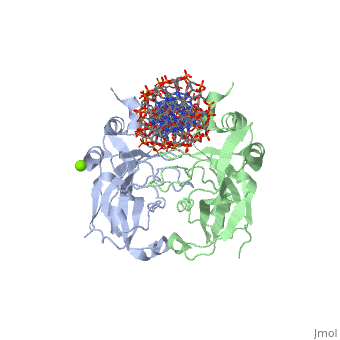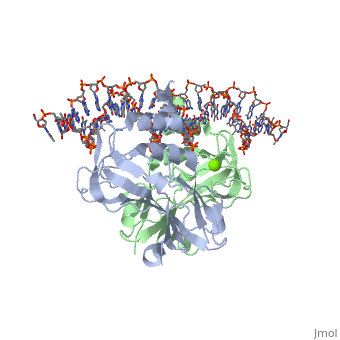TBX3: Difference between revisions
Helen Ginn (talk | contribs) adding green link |
David Canner (talk | contribs) No edit summary |
||
| Line 20: | Line 20: | ||
The helix 3<sub>10</sub>C, also present in TBX5, contacts DNA in the minor groove and hence widens it. However, overall the DNA is not bent, as seen in other crystal structures of [[T-box proteins]]. The 3<sub>10</sub> helix interacts with DNA using <scene name='User:Helen_Ginn/310c_277-279-281-283/1'>residues N277 (of the preceding loop), F279, K281 and F283.</scene> (See <scene name='User:Helen_Ginn/310c_277/3'>N277 only</scene>, <scene name='User:Helen_Ginn/310c_279/1'>F279 only</scene>, | The helix 3<sub>10</sub>C, also present in TBX5, contacts DNA in the minor groove and hence widens it. However, overall the DNA is not bent, as seen in other crystal structures of [[T-box proteins]]. The 3<sub>10</sub> helix interacts with DNA using <scene name='User:Helen_Ginn/310c_277-279-281-283/1'>residues N277 (of the preceding loop), F279, K281 and F283.</scene> (See <scene name='User:Helen_Ginn/310c_277/3'>N277 only</scene>, <scene name='User:Helen_Ginn/310c_279/1'>F279 only</scene>, | ||
<scene name='User:Helen_Ginn/310c_281/1'>F281 only</scene> and <scene name='User:Helen_Ginn/310c_283/1'>F283 only</scene>). | <scene name='User:Helen_Ginn/310c_281/1'>F281 only</scene> and <scene name='User:Helen_Ginn/310c_283/1'>F283 only</scene>). | ||
==Additional Resources== | |||
For additional information, see: [[Transcription and RNA Processing]] | |||
<br /> | |||
Revision as of 15:02, 2 October 2010
IntroductionIntroduction
TBX3 acts as a repressor and has had its crystal structure solved to 1.7 Å resolution. There are many differences between the solved structure of TBX3 and its homologue, Brachyury. Secondary structures and quaternary contacts are slightly different. TBX3 independently recognises two halves of a palindromic site whereas Brachyury is stabilised via interactions between the two monomers. Despite this, the T-box domain of TBX3 and the T-box domain of Brachyury can be superimposed with a rmsd of 1.3 Å for 167 atoms.
Crystal structureCrystal structure
TBX3 expressed from residues 101 - 291 were viable proteins and were crystallised in space group P212121. These included two monomers and one DNA duplex in the asymmetric unit. The monomers themselves can be superimposed to give an rmsd of 0.5 Å. The largest differences between the TBX3 crystal structure and the T-box domain of Brachyury is between residues 173 and 184, and between strands F and G. Four residues are inserted between F and G and this adopts a 310 conformation.
Interaction with DNAInteraction with DNA
Loops that protrude from one end of the β barrel and the C-terminal helical extension contact both the major and minor grooves of the DNA. Strand B contains residues , and recognises the major groove. Arg130 is the only residue in direct contact with a DNA base in the major groove and recognises N7 and H2O.O6 of guanine (base pair 5) and the corresponding phosphate. Arg131 interacts with the DNA backbone mediated by water.
gα3 loop interaction with DNAgα3 loop interaction with DNA
The gα3 loop spans the major groove. H3 (Tyr264, Gln265 and ) form polar contacts to the DNA backbone and hydrophobic contacts to the ribose moiety. There are similar contacts in the crystal structure of Brachyury.
310 helix interaction with DNA310 helix interaction with DNA
The helix 310C, also present in TBX5, contacts DNA in the minor groove and hence widens it. However, overall the DNA is not bent, as seen in other crystal structures of T-box proteins. The 310 helix interacts with DNA using (See , , and ).
Additional ResourcesAdditional Resources
For additional information, see: Transcription and RNA Processing