Citrate Synthase: Difference between revisions
| Line 1: | Line 1: | ||
==The Structure and Mechanism of Citrate Synthase== | ==The Structure and Mechanism of Citrate Synthase== | ||
<applet load='2cts' size='300' color='white' frame='true' align='right' caption='Citrate Synthase Closed Form' name='closed'/> <applet load='1cts' size='300' color='white' frame='true' align='right' caption='Citrate Synthase Open Form' name='open'/> | <applet load='2cts' size='300' color='white' frame='true' align='right' caption='Citrate Synthase Closed Form' name='closed'/> <applet load='1cts' size='300' color='white' frame='true' align='right' caption='Citrate Synthase Open Form' name='open'/> | ||
Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme (in fact, it is often used as a quantitative enzyme marker for intact mitochondria), it is encoded by nuclear DNA, not mitochondrial <ref>"Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010. <http://en.wikipedia.org/wiki/Citrate_synthase></ref>. The standard free energy change (ΔG°’) for the citrate synthase reaction is | Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme (in fact, it is often used as a quantitative enzyme marker for intact mitochondria), it is encoded by nuclear DNA, not mitochondrial <ref>"Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010. <http://en.wikipedia.org/wiki/Citrate_synthase></ref>. The standard free energy change (ΔG°’) for the citrate synthase reaction is | ||
-31.5kJ/mol <ref name="voet">Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.</ref>. This negative free energy value means that citrate synthase is likely to function far from equilibrium under physiological conditions, and is thus a rate-determining enzyme in the citric acid cycle. | |||
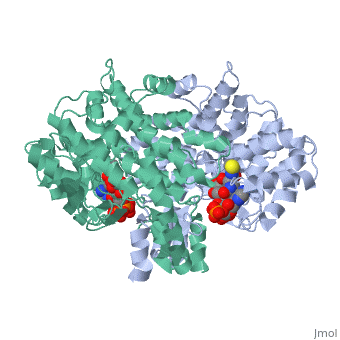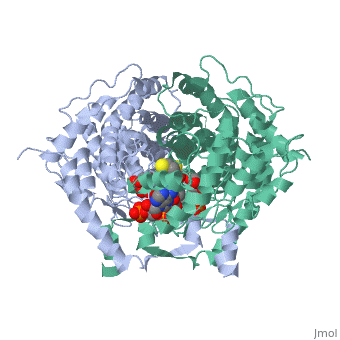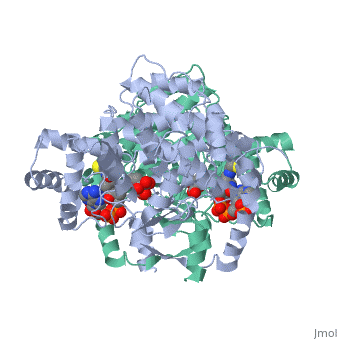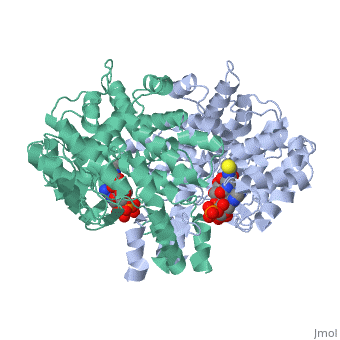
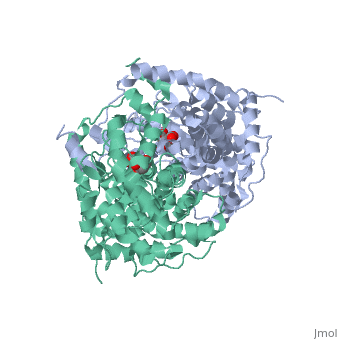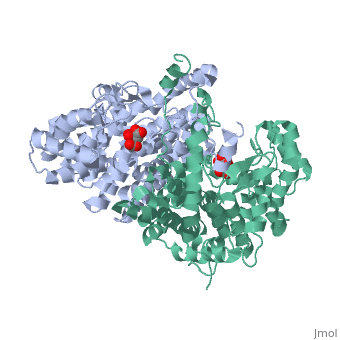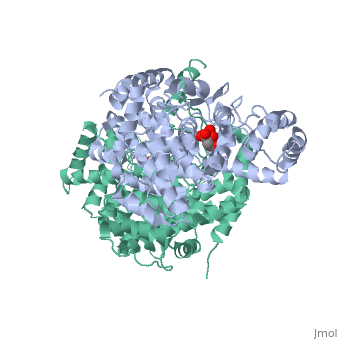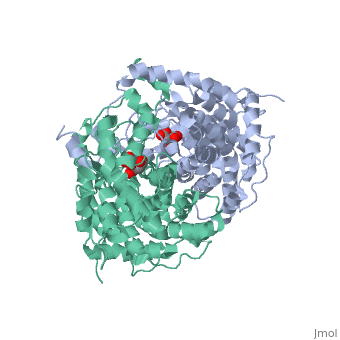
'''Structure:''' Citrate synthase is a single amino acid chain <scene name='Daniel_Eddelman_Sandbox_2/Cts_open_monomer/1'>monomer</scene>. Biologically, however, it exists as a | '''Structure:''' Citrate synthase is a single amino acid chain <scene name='Daniel_Eddelman_Sandbox_2/Cts_open_monomer/1'>monomer</scene>. Biologically, however, it exists as a | ||
Revision as of 10:59, 22 March 2010
The Structure and Mechanism of Citrate SynthaseThe Structure and Mechanism of Citrate Synthase
|
|
Citrate synthase is an enzyme active in the mitochondria, where it is responsible for catalyzing the first reaction of the citric acid cycle (Krebs Cycle): the condensation of acetyl-CoA and oxaloacetate to form citrate. Although it is a mitochondrial enzyme (in fact, it is often used as a quantitative enzyme marker for intact mitochondria), it is encoded by nuclear DNA, not mitochondrial [1]. The standard free energy change (ΔG°’) for the citrate synthase reaction is -31.5kJ/mol [2]. This negative free energy value means that citrate synthase is likely to function far from equilibrium under physiological conditions, and is thus a rate-determining enzyme in the citric acid cycle.
Structure: Citrate synthase is a single amino acid chain . Biologically, however, it exists as a . Each identical subunit consists of a large and a small domain, and is comprised almost entirely of α helices (making it an all α protein). In its free enzyme state, citrate synthase exists in “open” form, with its two domains forming a cleft containing the substrate (oxaloacetate) binding site (PDB: 1cts) [3]. When oxaloacetate binds, the smaller domain undergoes an 18° rotation, sealing the oxaloacetate binding site and resulting in the (PDB: 2cts). This conformational change not only prevents solvent from reaching the bound substrate, but also generates the acetyl-CoA binding site. This presence of “open” and “closed” forms results in citrate synthase having Ordered Sequential kinetic behavior [2].
Mechanism: The reaction mechanism for citrate synthase was proposed by James Remington. In this mechanism, three ionizable side chains in the of citrate synthase participate in acid-base catalysis: His 274, His 320, and Asp 375. First, (a base) removes a proton from the methyl group of acetyl-CoA to form its enol. stabilizes the acetyl-CoA enolate by forming a hydrogen bond with the enolate oxygen. The enolate then nucleophilically attacks oxaloacetate’s carbonyl carbon, and donates a proton to oxaloacetate’s carbonyl group in a concerted step, forming citryl-CoA (which remains bound to the enzyme). Finally, citryl-CoA is hydrolyzed to citrate and CoA.
Regulation: Perhaps the most crucial regulators of the citrate synthase reaction are its substrates, acetyl-CoA and oxaloacetate. Both are present in the mitochondria at concentrations below saturation of citrate synthase. The metabolic flux is controlled by substrate availability, so controlling the levels of acetyl-CoA and oxaloacetate in the mitochondria controls the rate of reaction. Furthermore, citrate synthase is inhibited by NADH, citrate (which competes with oxaloacetate), and succinyl-CoA (an example of competitive feedback inhibition) [4].
- ↑ "Citrate Synthase -." Wikipedia, the Free Encyclopedia. Web. 22 Mar. 2010. <http://en.wikipedia.org/wiki/Citrate_synthase>
- ↑ 2.0 2.1 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry: Life at the Molecular Level. Hoboken, NJ: Wiley, 2008.
- ↑ Remington S, Wiegand G, Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111-52. PMID:7120407
- ↑ Wiegand G, Remington SJ. Citrate synthase: structure, control, and mechanism. Annu Rev Biophys Biophys Chem. 1986;15:97-117. PMID:3013232 doi:http://dx.doi.org/10.1146/annurev.bb.15.060186.000525